Atomic Structure
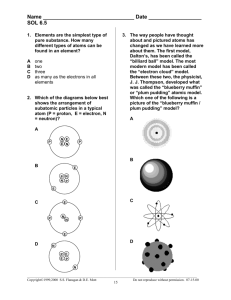
What are the 3 major parts of an atom?
What are the 3 major parts of an atom?
Proton
Neutron
Electron
Parts of an Atom
Another Model
SIMPLIFIED MODEL.
PROTON
Protons are positively charged particles found in the atomic nucleus. Protons were discovered by Ernest Rutherford..
Experiments done in the late 1960's and early 1970's showed that protons are made from other particles called quarks.
Protons are made from two 'up' quarks and one 'down' quark.
NEUTRON
Neutrons are uncharged particles found in the atomic nucleus. Neutrons were discovered by James Chadwick in 1932.
Experiments done in the late 1960's and early 1970's showed that neutrons are made from other particles called quarks.
Neutrons are made from one 'up' quark and two 'down' quarks.
ELECTRON
Electrons are negatively charged particles that surround the atom's nucleus.
Electrons were discovered by J. J.
Thomson in 1897.
Electrons determine properties of the atom. Chemical reactions involve sharing or exchanging electrons.
NUCLEUS
The nucleus is the central part of an atom.
It is composed of protons and neutrons.
The nucleus contains most of an atom's mass.
It was discovered by Ernest Rutherford in
1911.
ATOMIC MASS
Atomic Mass is the total mass of the
Protons and Neutrons. Electrons are not measured in the mass.
The atomic mass number found on the periodic table is the average mass of all the isotopes of a given atom.
Quarks
Believed to be one of the basic building blocks of matter.(part of an atom) Quarks were first discovered in experiments done in the late
1960's and early 1970's.
Three families of quarks are known to exist. Each family contains two quarks. The first family consists of Up and Down quarks, the quarks that join together to form protons and neutrons.
The second family consists of Strange and Charm quarks and only exist at high energies.
The third family consists of Top and Bottom quarks and only exist at very high energies.
Isotope
ISOTOPE- Atoms that have the same number of protons but different numbers of neutrons. Occur both naturally and synthetically.
Isotope models
Please draw and label these models in your journal.
What is the Electron Cloud Model?
Model of the atom that pictures the electrons moving around the nucleus in a region called an electron cloud.
The electron cloud is a cloud of varying density surrounding the nucleus. The varying density shows where an electron is more or less likely to be. Atoms with electrons in higher energy levels have additional electron clouds of different shapes that also show where those electrons are likely to be.
Electron Cloud Model
Diagram 1:
Electron Cloud Model
Diagram 2:
Valence Electron-
valence electrons are found in the outermost “shell”, or ring of the atom. In these models you can see that Lithium has one valence electron, while Carbon has four.