si worksheet 3
advertisement

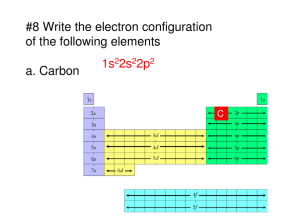
SI WORKSHEET 3 1. Why are electrons lost from the s block before the d block on transition metal elements? Electrons in the s block are higher in energy once the d block has electrons and so they are more easily lost from the transition metals 2. Give the condensed and noble gas notation for Iron. What is the noble gas notation for Fe2+ and Fe3+? Which would you say is more stable? Fe2+ = [Ar]3d6 Fe3+= [Ar]3d5 Fe3+ has a half filled subshell so it is more stable. 3. What is the noble gas notation for Ca2t? What about S2-? (Do you see how they are related?) Both have the noble gas notation for Argon, different elements can have the same electron configurations as noble gases 4. What is the condensed electron configuration for Tin in its ionic states? Tin usually forms a +2 charge. Knowing that I can find its electron configuration when it has a +2 charge. Tin can also form a +4 charge. We can determine this from its electron configuration Sn0 =1s22s22p63s23p64s23d104p65s24d105p2. Loss of the 2 5p electrons= Sn2+1s22s22p63s23p64s23d104p65s24d10. Loss of the 2 5s electrons= Sn4+1s22s22p63s23p64s23d104p64d10 5. Which is the biggest atom: Oxygen, Sulfur, Tellurium, or Selenium? Tellurium 6. Based on the periodic table what atom would be the biggest atom on the table? What about the smallest? Francium is the biggest and Helium would be the smallest 7. Order these in order of increasing radii: C, Br, Se2-, P, F-, S. C, F-, S, P, Br, Se2- 8. Which would have a higher electron affinity: Fluorine or Sodium? Which has a higher first ionization energy? Fluorine has the higher electron affinity (more negative) and Sodium has he higher first ionization energy (more positive) 9. Why do noble gases and nitrogen have a positive electron affinity while most other atoms have negative electron affinities? The have fully filled or half-filled subshells which are stable. Adding an electron to a fully filled or half-filled subshell disrupts this stability.