#8 Write the electron configuration of the following elements a
advertisement
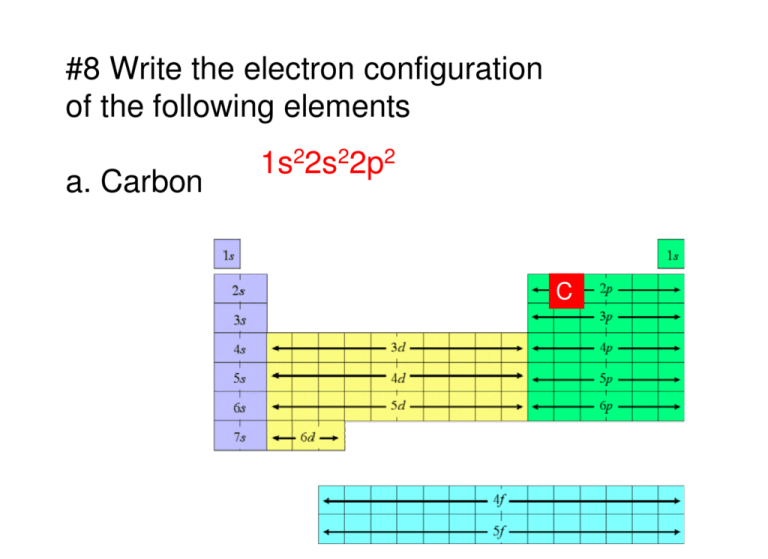
#8 Write the electron configuration of the following elements a. Carbon 1s22s22p2 C #8 Write the electron configuration of the following elements b. strontium 1s22s22p23s23p64s23d104p65s2 Sr #8 Write the electron configuration of the following elements c. Vanadium 1s22s22p23s23p64s23d3 V #9 List the symbols for all the elements whose electron configuration ends as follows: Each n represents an energy level. a. ns2np1 B Al Ga In Tl 1 2 3 4 5 #9 List the symbols for all the elements whose electron configuration ends as follows: Each n represents an energy level. b. ns2np5 F Cl Br I At 1 2 3 4 5 #9 List the symbols for all the elements whose electron configuration ends as follows: Each n represents an energy level. c. (n+1)s2nd2 Ti Zr Hf Rf 1 1 2 3 4 5 6 7 8 9 10 2 3 4 5 #10 What information can be included in a periodic table? Answer: All kinds of data about the nature and properties of the elements and their relationship to one another. The major trends in elements can be predicted because of the order inherent in the table. #11 Into what four classes can elements be sorted based on their electron configuration? And Noble Gases #12 Why do the elements potassium and sodium have similar chemical properties? High Energy s orbital ONE electron #13 Classify each element as a representative element, transition metal, or noble gas. 1s22s22p63s23p64s23d104p6 #13 Classify each element as a representative element, transition metal, or noble gas. 1s22s22p63s23p64s23d6 #13 Classify each element as a representative element, transition metal, or noble gas. 1s22s22p63s23p2 #14 Which of the following elements are transition metals? Cu Sr Cd Au Al Ge Co http://wps.prenhall.com/wps/media/objects/476/488316/ch09.html #15 How many electrons are in the highest occupied energy levels of a Group 5A element? ns2np3 Therefore they all have 5 electrons in the highest energy level