Lewis Dot structure
advertisement
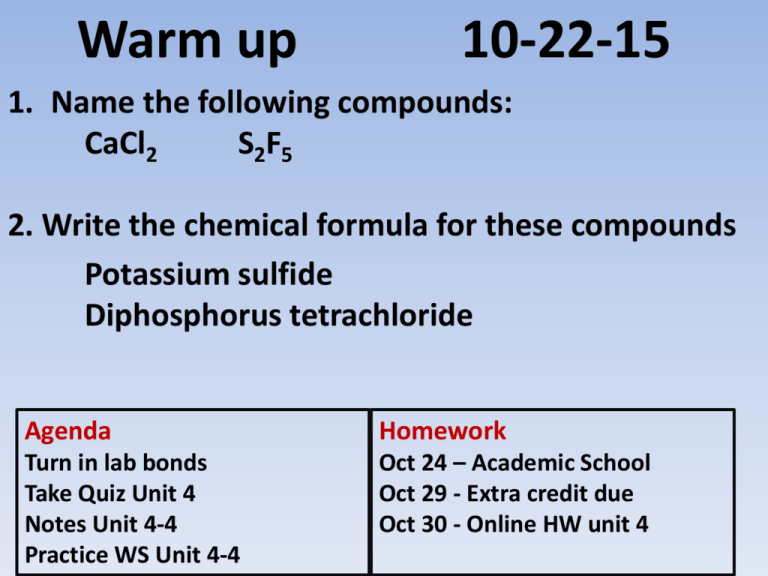
Warm up 10-22-15 1. Name the following compounds: CaCl2 S2F5 2. Write the chemical formula for these compounds Potassium sulfide Diphosphorus tetrachloride Agenda Homework Turn in lab bonds Take Quiz Unit 4 Notes Unit 4-4 Practice WS Unit 4-4 Oct 24 – Academic School Oct 29 - Extra credit due Oct 30 - Online HW unit 4 Quiz Unit 4 2 questions - Finding Ions (positive and negative charge) 2 questions - Bonds (3 types) 2 questions - Naming compounds (rule 1 & 2) You will need a half sheet of paper Record your quiz version # Unit 4-4 Lewis Dot structure How do we determine which elements will combine to make bonds? Nomenclature: Ionic Compounds Transition Metal Examples Regular metal 1. 2. 3. 4. CaCl2 = Calcium chloride Na2S = Sodium sulfide FeCl2 FeN Iron (I) nitride Iron (II) nitride Answers 1. Iron (II) chloride 2. Iron (III) nitride 3. Fe3N 4. Fe3N2 Nomenclature: Ionic Compounds Polyatomic Ions (NH4)+ (OH)(CO3)-2 (NO3)(SO4)-2 (PO4 )-3 (Cr2O7)-2 Ammonium Hydroxide Carbonate Nitrate Sulfate Phosphate Dichromate (NH3) Ammonia (NO2)(SO3)-2 (PO3)-3 Nitrite Sulfite Phosphite Naming Polyatomic Ions Examples 1. 2. 3. 4. ______________ Strontium sulfate Calcium nitrite ______________ Ammonium Sulfide Potassium hydroxide SrSO4 Ca(NO2)2 (NH 4)2S _____ KOH _____ Naming Ionic Compounds Practice 1. 2. 3. 4. 5. 6. 7. 8. ______________ ______________ Lithium Bromide Calcium Fluoride ____________ Iron (III) Phosphide ______________ ______________ AlCl3 Na2S _____ _____ CuCl2 Transition metal ____ Li3PO4 Sr(NO3)2 Electron Distribution in Molecules G. N. Lewis 1875 - 1946 • Electron distribution is depicted with Lewis structures (electron dot) showing valence electrons • Show the number of bond in the covalent compound (In ionic bonds, it was decided with charges) Lewis Structures for Elements 1) Write the element symbol. 2) Starting at the right, draw 4 electrons, or dots, one on each side around the element symbol. Lewis Structures Try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Bond Formation A bond can result from an overlap of atomic orbitals on neighboring atoms. •• H + Cl •• •• • • H Cl •• • • •• H Cl Note that each atom has a single, unpaired electron. •• • • Bond and Lone Pairs • Valence electrons are distributed as shared or BOND PAIRS and unshared or LONE PAIRS. •• H • • Cl • • •• shared or bond pair lone pair (LP) This is called a LEWIS structure. Sharing Electrons • All atoms want to have 8 valence electrons (except H, only needs 2) • Obtain a total of 8 by sharing • Only lonely electrons will share – pair of electrons with existing partners will not share and make bonds Types of Bonds Single bond Cl2 Double bond = CO2 Triple bond N2 Example Lewis Dot for Compounds Draw CH4 Draw C2H4 Draw H2O2 Valence Shell Electron Pair Repulsion (VSEPR) • Lewis structure help predict molecular shape Assignments • Summary • Lewis dot worksheet (4-4) due next class





