5 Ksp problems
advertisement

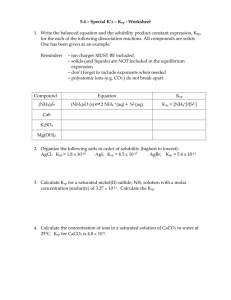
The equilibrium product constant A salt is considered soluble if it dissolves in water to give a solution with a concentration of at least 0.1 M at room temperature. A salt is considered insoluble if the concentration of an aqueous solution is less than 0.0001 M at room temperature. Salts with solubilities between 0.0001 M and 0.1 M are considered to be slightly soluble. Can a particular we want some things to be in solution and some things to settle… chlorine is nice to have out of water, but we don’t want lead to settle. compound be added to a lake to kill organisms /plants /fish, yet not ppt to the bottom? #5 will be homework 1. Write the dissociation of each salt, so that you know want the initial amount is. 2. Calculate the concentration of the PbCl2 3. Look at the Ksp chart and see if the concentration is stronger (the power of 10 is > than the Ksp – which means less negative.) 1. Pb(NO3)2 Pb+2 + 2NO3FeCl2 Fe+2 + 2Cl 2. Ksp of the lead chloride = [Pb+2] [2Cl-]2 = [.01] [2 x .06]2 *you need to have .06 since the original dissociation was 2 x .03M… This calculates out to [.01] [.0144] = = .00014 = 1.44 x 10-4 The Ksp = 1.7 x 10-5, so there will be a ppt since the answer is stronger There will be answers to it tomorrow in class. An experiment calls for 22.5g of Lead (II) Chloride dissolved in 500 mL of distilled water. Is this possible? Calculate the Ksp at room temperature and compare to the actual. What could you do to dissolve this salt if 22.5 g saturates the solution? The formula for lead(II) chloride is : PbCl2 The formula mass is 207 + 35.5 + 35.5 = 278g 22.5g x 1 mole = .0809 moles 278g The concentration or molarity is: 0809mo. = .162M .500L Ksp comes from the dissociation equation which is… PbCl2 Pb+2 + 2Cl-1 Ksp = [.162]1 [ 2 x .162]2 = (.162) (.105) = .0170 = 1.70 x 10 -2 Why does Hoffmann Always make one More step Of work? There is one final step that we need to recognize and understand….regarding the ions that have a coefficient greater that 1 in the balanced equation. Similar to Keq, we need to square or cube the concentration; but there is one more step. (What’s new??) We must recognize that the coefficient NOT ONLY is acknowledged in the superscript, but is also recognized as an amount of twice or three times the number of ions as follows: The concentration of each of the three Parts is .162 So the Keq would actually be: (.162) (2 x .162)2 = Pb+2 .0170 -1 Cl This Is a much greater -1 Cl Value than the .00425 Try this qualitative problem: If sodium bromide were added to the beaker, what would settle out? If this(these) ppt. were separated from the solution of the other ions and at this point, and OH- were added to the solution, what would settle? After separation of this(these) ppt. potassium carbonate is added to the solution. What settles? CuBr Mg(OH)2 Fe(OH)2 BaCO3 A paragraph fill in the blank using vocabulary terms correctly. 2. A qualitative precipitate in a f low chart problem. 3. A quantitative Ksp problem similar to yesterday’s work with given concentrations like #5 and #6. 4. A quantitative Ksp problem that you need to calculate if given concentrations 1. https://www.youtube.com/watch?v=u1oPVBPU0wk https://www.youtube.com/watch?v=u1oPVBPU0wk (go to 6 min on the second one) http://www.scienceiscool.org/ksp/tutorials.html http://chemed.chem.purdue.edu/genchem/topicrevie w/bp/ch18/ksp.php http://www.chemteam.info/Equilibrium/Calc-Ksp- FromMolSolub.html