Solubility Equilibrium Practice Problems
advertisement

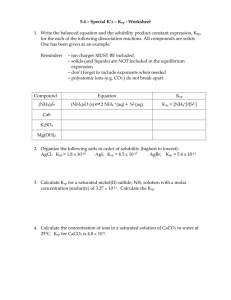
1. 2. Practice Problems: Solubility Equilibrium Calculations Write the solubility product expression for Ag2CO3. What is the concentration of lead ions and sulfide ions in a saturated solution of lead sulfide (PbS) solution at 25oC? Ksp = 3.0x10-28 Answer: 2x10-14 M 3. The Ksp of barium sulfate is 1.1x10-10. What is the sulfate-ion concentration of a 1.00L saturated solution of BaSO4 to which 0.015 mol of Ba(NO3)2 is added? *Write out the solubility equation for Ba(NO3)2. Answer: 7.3x10-9 M 4. Explain which compound below is more soluble in water. a. 5. FeS: Ksp = 8.0x10-19 and CuS: Ksp = 8.0x10-37 The Ksp of SrSO4 is 3.2x10-7. What is the equilibrium concentration of the sulfate ion in a 1.0-L solution of strontium sulfate to which 0.10 mol of Sr(CH3CO2)2 has been added? Answer: 3.2x10-6 M 6. Calculate the solubility of cadmium sulfide, CdS, in mol/L given the Ksp value for CdS is 8.0x10-27 Answer: 8.9x10-14 mol/L 7. A 5.0 gram sample of Ag2SO4 will dissolve in 1.0L of water. Calculate the solubility product constant for this salt. Answer: 1.6x10-5 8. Calculate the solubility product constant, Ksp, for copper (I) chloride, given that the solubility of this compound at 25oC is 1.08x10-2g/100g H2O. Answer: 1.19x10-6 9. What is the concentration of iodide ions in a saturated solution of lead iodide (PbI2) at 25.0oC? Ksp = 7.9x10-9. Answer: I- = 0.00252M ; Pb2+ = 0.00126M 10. Would precipitation occur when 500mL of a 0.02M solution of AgNO3 is mixed with 500mL of 0.001M solution of NaCl? Explain your answer. Answer: yes,AgCl because the ions product = 1x10-4 > Ksp of AgCl = 8.3x10-17 11. Predict whether calcium carbonate will precipitate when 0.5L of 0.001 M Ca(NO3)2 is mixed with 0.5L of 0.0008 M Na2CO3 to form 1.0L of solution. The Ksp of CaCO3 is 4.5x10-9. Yes, CaCO3 ion product = 2x10-7> Ksp of CaCO3 = 4.5x10-9