Unit 2 Equilibrium and Ksp
advertisement

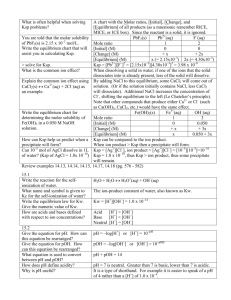
Jonathan Versino Peter Lansky Unit 2 Equilibrium and Ksp AxBy (s) xAy+ (aq) + yBx- (aq) -Equilibrium constant = K = [Ay+]x[Bx-]y / [AB] -Solubility product constant = Ksp = [Ay+]x [Bx-]y Le Chatelier’s principal – “a change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner as to reduce or counteract the effect of the change.” Reaction Quotient = Q = [products] / [reactants] Q is used for a chemical reaction that is doesn’t have to be at equilibrium. If Q < Ksp then reactants will be converted to products. If Q = Ksp the system is at equilibrium. If Q > Ksp some products will be converted to reactants Common ion effect – When excess ions are added to the equilibrium it will lower the salt solubility. -10 If, Ksp = [Ag+][Cl-] = 2.5x10 Then, (x)(x) = 2.5x10-10 Then, x = [Ag+] = [Cl-] = 1.6x10-5 So,if 0.10 mol of NaCl is added to 1 L of the AgCl solution Then, [Ag+] [0.1M] = 2.5x10-10 And [Ag+] = 2.5x1-9 Solubility rules -Almost all salts of Na+, K+, andNH4+ -All salts of Cl-, Br-, and I- except Halides of Ag+, Hg22+, and Pb2+ -Compounds containing F- except Fluorides of Mg2+, Ca2+, Sr2+, Ba2+, Pb2+ -Salts of NO3-, ClO3-, ClO4-, CH3CO2-Salts of SO42Colligative Properties -The properties of a solution that are dependant only on the number of solute particles per solvent molecule -Molality = moles of solute/kilogram of solvent Freezing Point Depression -Freezing point of a solution is lowered with the addition of a solute, usually quite small ∆Tf =Kfm ∆Tf = freezing point depression; m=molality, Kf=molal freezing point constant Boiling Point Elevation -Adding a solute to a solution increases the temperature at which it boils ∆Tb =Kbm Metallic and Ionic Solids Structure -Simple Cubic • 1 net atom per unit cell • Cell edge = 2r -Body Centered Cubic • 2 atom per unit cell • Cell diagonal = 4r = (√3)(Length of Edge) -Face Centered Cubic • 4 net atoms per unit cell • Cell diagonal = (√2)(length of edge)