Homework_6_Su15
advertisement

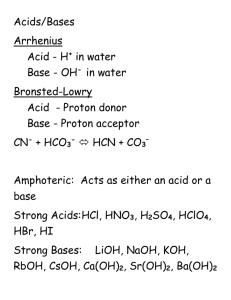
Name ____________________________ Homework #6 Chem V01B Summer 2015 Ionic Equilibria 1. A 10.0 mL sample of 1.00 M HNO2 (pKa = 3.34 and Ka = 4.57x10-4) is titrated with 1.00 M NaOH. a. What is the initial pH (before any NaOH is added) (6 marks) b. What is the pH at the half equivalence point (6 marks) c. What is the pH of the solution when 10.0 mL of the NaOH is added (6 marks)? 2. Calculate the solubility of PbCl2 (Ksp = 1.7x10-5) 3. Calculate the solubility of Mg(OH)2 (Ksp = 2.06x10-13) 4. Calculate the solubility of PbCl2 (Ksp = 1.7x10-5) in 0.200M NaCl 5. Consider the equilibrium a. Calculate the solubility of Mg(OH)2 (Ksp = 2.06x10-13) (6 marks) b. Calculate the solubility of Mg(OH)2 (Ksp = 2.06x10-13) in 0.200M NaOH (6 marks) c. Does the addition of the NaOH increase or decrease the solubility? (1 mark) d. There is a name for this effect what is it? (1 mark) 6. You have a sample of seawater in which the [Mg2+] = 0.069M and [Ca2+] = 0.0080M a. What is the minimum [OH−] necessary to just begin to precipitate Mg2+ from seawater assuming for Mg(OH)2 Ksp=2.06x10-13)? b. What is the [Mg2+] when Ca2+ just begins to precipitate from seawater (assume Ca(OH)2 Ksp=4.68x10-6)? 7. A 0.50 g sample of AgCl (Ksp = 1.77x10-10) is shaken with 5.0 mL of 6.0 M NH3 until there is no more reaction. The ammonia can react with Ag+(aq) to make a di-ammine silver (I) ion a. write a net ionic reaction that can happen b. calculate the mass of the AgCl that will remain 8. In the dead sea there is an astonishing 45.9 g/L of Mg2+ in the water. What is the minimum [OH−] necessary to just begin to precipitate Mg2+ from seawater assuming Ksp=2.06x10-13)?