amines reactions
advertisement

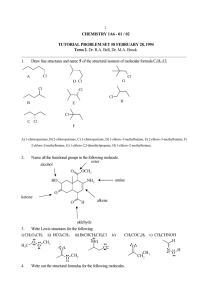
Amines, reactions Amines are similar to ammonia in their reactions. Like ammonia, amines are basic. Like ammonia, amines are nucleophilic and react with alkyl halides, acid chlorides, and carbonyl compounds. The aromatic amines are highly reactive in electrophilic aromatic substitution. Amine, reactions: 1. As bases 2. Alkylation 3. Reductive amination 4. Conversion into amides 5. EAS 6. Hofmann elimination from quarternary ammonium salts 7. Reactions with nitrous acid 1. As bases a) with acids b) relative base strength c) Kb d) effect of groups on base strength with acids NH2 + HCl NH3+Clanilinium chloride (CH3CH2)2NH + CH3COOH (CH3CH2)2NH2+, -OOCCH3 diethylammonium acetate relative base strength RNH2 > NH3 > ArNH2 Kb ionization of the base in water :Base + H2O H:Base+ + OH- Kb = [ H:Base+ ] [ OH- ] / [ :Base ] Kb aliphatic amines 10-3 – 10-4 ammonia 1.8 x 10-5 anilines 10-9 or less Why are aliphatic amines more basic than ammonia? NH3 + H2O NH4+ + OHR-NH2 + H2O R-NH3+ + OH- The alkyl group, -R, is an electron donating group. The donation of electrons helps to stabilize the ammonium ion by decreasing the positive charge, lowering the ΔH, shifting the ionization farther to the right and increasing the basicity. Why are aromatic amines less basic than aliphatic amines? R-NH2 + H2O R-NH3+ + OHNH3 NH2 + H2O NH2 NH2 + OH NH2 NH2 NH3 NH2 NH3 resonance stabilization of the free base, increases the ΔH, shifts the ionization to the left, decreasing base strength. Effect of substituent groups on base strength: NH2 NH3 + H2O G + OH G Electron donating groups will stabilize the anilinium ion, decreasing the ΔH, shifting the ionization farther to the right and making the compound a stronger base. Electron withdrawing groups destabilize the anilinium ion, increasing the ΔH, shifting the ionization towards the reactants, making the compound a weaker base. Common substituent groups: -NH2, -NHR, -NR2 -OH -OR -NHCOCH3 -C6H5 -R -H -X -CHO, -COR -SO3H -COOH, -COOR -CN -NR3+ -NO2 electron donating groups electron withdrawing groups Number the following in decreasing order of base strength (let #1 = most basic, etc. NH2 NH2 NH2 NH2 NH3 OCH3 NO2 4 1 5 3 2 2. Alkylation (ammonolysis of alkyl halides) RNH2 1o R-X R2NH R-X R3N 3o 2o R-X R4N+X4o salt SN2: R-X must be 1o or CH3 CH3CH2CH2CH2Br NH3 CH3CH2CH2CH2NH2 n-butylamine CH3CH2CH2NH2 n-propylamine NH2 CH3Cl CH3CH2CH2NHCH3 methyl-n-propylamine 2 CH3CH2Br aniline Et N Et N,N-diethylaniline (xs) CH3I H2 C NH2 benzylamine H2 CH3 C N CH3 CH3 I benzyltrimethylammonium iodide 3. Reductive amination C O + RNH2 C O + R2NH H2, Ni CH NHR 2o amine CH NR2 3o amine or NaBH3CN H2, Ni or NaBH3CN O CCH2CH3 + CH3CH2NH2 propiophenone O cyclohexanone NaBH3CN CH2CH3 NH CHCH2CH3 1-(N-ethylamino)-1-phenylpropane CH3NH2, H2/Ni NHCH3 cyclohexylmethylamine 4. Conversion into amides R-NH2 + RCOCl RCONHR + HCl 1o N-subst. amide R2NH + RCOCl RCONR2 + HCl 2o R3N 3o N,N-disubst. amide + RCOCl NR NH2 O H N C CH3 + (CH3CO)2O N-phenylacetamide O C Cl (CH3CH2)2NH + H3C H3C O C N CH2CH3 CH2CH3 N.N-diethyl-m-toluamide DEET O N CH3 CH3 + CH3C Cl NR Conversion into sulfonamides R-NH2 + ArSO2Cl ArSO2NHR + HCl 1o N-subst.sulfonamide R2NH + ArSO2Cl ArSO2NR2 + HCl 2o R3N N,N-disubst.sufonamide + ArSO2Cl NR Schotten-Baumann technique: reactions of aromatic acid chlorides are sped up by the addition of base. R-NH2 + ArSO2Cl + KOH ArSO2NHR 1o acidic ArSO2NR water soluble salt R2NH + ArSO2Cl + KOH ArSO2NR2 + HCl 2o N,N-disubst.sufonamide water insoluble Hinsberg Test: unknown amine + benzenesulfonyl chloride, KOH (aq) Reacts to produce a clear solution and then gives a ppt upon acidification primary amine. Reacts to produce a ppt secondary amine. Doesn’t react tertiary amine. O S O KOH NH2 + SO2Cl N water sol. O CH2CH3 S N CH2CH3 O KOH (CH3CH2)2NH + SO2Cl ppt KOH N CH3 CH3 + SO2Cl NR sulfanilamide “magic bullet” NH2 SO2 NH2 antibiotic OH N N H2N N H2 H C N O H COOH C N CH CH2CH2COOH N folic acid H2N COOH p-aminobenzoic acd H2N SO2NH2 sulfanilamide 5. EAS -NH2, -NHR, -NR2 are powerful activating groups and ortho/para directors a) nitration b) sulfonation c) halogenation d) Friedel-Crafts alkylation e) Friedel-Crafts acylation f) coupling with diazonium salts g) nitrosation a) nitration NH2 HNO3 TAR! H2SO4 (CH3CO)2O NHCOCH3 NH2 NHCOCH3 HNO3 H2O,OH- H2SO4 NO2 + ortho- NO2 b) sulfonation NH3 NH2 + H2SO4 SO3 cold H2SO4 NH3 HSO4 c) halogenation NH2 NH2 Br Br polyhalogenation! + Br2, aq. Br no catalyst needed use polar solvent Br Br2,Fe Br HNO3 Br H2/Ni H2SO4 NO2 + ortho- NH2 Swimming pool test kit for chlorine: NH2 CH3 o-toluidine NH2 Cl2 (aq.) Cl CH3 Cl bright yellow! e) Friedel-Crafts alkylation NR with –NH2, -NHR, -NR2 NH2 CH3 + CH3CH2Br, AlCl3 NR Do not confuse the above with the alkylation reaction: NH2 NHCH2CH3 CH3 CH3 + CH3CH2Br f) Friedel-Crafts acylation NR with –NH2, -NHR, -NR2 NH2 CH3 + O H3C C Cl AlCl3 NR Do not confuse the above with the formation of amides: O NH2 NHCCH3 CH3 CH3 O + H3C C Cl g) nitrosation H3C N H3C CH3 N CH3 NaNO2, HCl O N The ring is sufficiently activated towards EAS to react with the weak electrophile NO+ h) coupling with diazonium salts azo dyes N2 Cl NH2 NH2 CH3 CH3 + benzenediazonium chloride an azo dye N N 6. Hofmann elimination from quarternary hydroxides step 1, exhaustive methylation 4o salt step 2, reaction with Ag2O 4o hydroxide + AgX step 3, heat to eliminate alkene(s) + R3N (xs) CH3I CH3 CH3CH2CH2CH2 N CH3 CH3 CH3CH2CH2CH2 NH2 CH3 CH3CH2CH2CH2 N CH3 CH3 I- CH3 CH3CH2CH2CH2 N CH3 OH CH3 Ag2O I- CH3 CH3CH2CH2CH2 N CH3 OH- + AgI CH3 CH3CH2CH=CH2 + (CH3)3N CH3CH2CHCH3 + (xs) CH3I NH2 CH3CH2CHCH3 H3C N CH3 CH3 CH3CH2CHCH3 H3C N CH3 CH3 I- OH Ag2O CH3CH2CHCH3 H3C N CH3 CH3 CH3CH2CHCH3 H3C N CH3 CH3 I- OH + AgI CH3CH2CH=CH2 + CH3CH=CHCH3 chief product + (CH3)3N Hofmann orientation 7. Reactions with nitrous acid primary amines R-NH2 N N + HONO NH2 + HONO diazonium salt N2 + mixture of alchols & alkenes secondary amines H N R O N N R + HONO N-nitrosamine tertiary amines N R + HONO R O N N R R p-nitrosocompound note: 90% of all tested nitrosamines are carcinogenic in man. Many nitrosamine cancers are organ specific. For example, dimethylnitrosamine causes liver cancer while the nitrosamines in tobacco smoke cause lung cancer. Sodium nitrite (“cure”) is used as a preservative in meats such as bacon, bologna, hot dogs, etc. to kill the organism responsible for botulism poisoning. In the stomach, the nitrous acid produced from sodium nitrite can react with secondary and tertiary amines to form nitrosamines. To reduce the formation of nitrosamines, ascorbic acid (Vitamin C) is now added to foods cured with sodium nitrite. Nitrosamines are also found in beer! Amines, reactions Amines are similar to ammonia in their reactions. Like ammonia, amines are basic. Like ammonia, amines are nucleophilic and react with alkyl halides, acid chlorides, and carbonyl compounds. The aromatic amines are highly reactive in electrophilic aromatic substitution. Amine, reactions: 1. As bases 2. Alkylation 3. Reductive amination 4. Conversion into amides 5. EAS 6. Hofmann elimination from quarternary ammonium salts 7. Reactions with nitrous acid