Chapter 19: Carboxylic Acids
advertisement

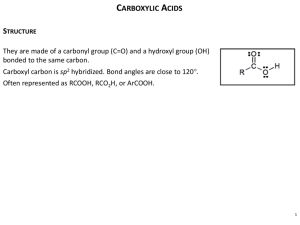
CHAPTER 14: CARBOXYLIC ACIDS INTRODUCTION The functional group of carboxylic acids consists of a C=O with -OH bonded to the same carbon. Carboxyl group is usually written -COOH. Aliphatic acids have an alkyl group bonded to COOH. Fatty acids are long-chain aliphatic acids Aromatic acids have an aryl group bonded to COOH. 2 COMMON NAMES Many aliphatic acids have historical names. Positions of substituents on the chain are labeled with Greek letters. 3 Ph Cl O CH3CH2CHC OH -chlorobutyric acid CH3CH2CH2CHCH2COOH -phenylcaproic acid NOMENCLATURE Common names you need to know: Formic acid (methanoic acid) O H OH Acetic Acid (ethanoic acid) O Benzoic Acid HO O OH Propionic Acid (propanoic acid) Butyric acid (butanoic acid) Valeric acid (pentanoic acid) IUPAC NAMES Remove -e from alkane (or alkene) name, add -oic acid. The carbon of the carboxyl group is #1. CH3CH2CHC OH Ph H H C C COOH 2-chlorobutanoic acid trans-3-phenyl-2-propenoic acid 7 Cl O Nomenclature • For acyclic acid chlorides: change the suffix –ic acid of the parent carboxylic acid to the suffix –yl chloride; or • When the –COCl group is bonded to a ring: change the suffix carboxylic acid to –carbonyl chloride. – NAMING CYCLIC ACIDS Cycloalkanes bonded to -COOH are named as cycloalkanecarboxylic acids. Aromatic acids are named as benzoic acids. CH(CH3)2 2-isopropylcyclopentanecarboxylic acid 9 COOH COOH OH o-hydroxybenzoic acid DICARBOXYLIC ACIDS Aliphatic diacids are usually called by their common names. For IUPAC name, number the chain from the end closest to a substituent. Two carboxyl groups on a benzene ring indicate a phthalic acid. HOOCCH2CHCH2CH2COOH 3-bromohexanedioic acid -bromoadipic acid (common) 10 Br STRUCTURE OF CARBOXYL Carbon is sp2 hybridized. Bond angles are close to 120. O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. BOILING POINTS Higher boiling points than similar alcohols, due to dimer formation. 12 Acetic acid, b.p. 118C PHYSICAL PROPERTIES MELTING POINTS Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis) lower the melting point. Note these 18-C acids: 14 Stearic acid (saturated): 72C Oleic acid (one cis double bond): 16C Linoleic acid (two cis double bonds): -5C SOLUBILITY Water solubility decreases with the length of the carbon chain. Up to 4 carbons, acid is miscible in water. More soluble in alcohol. Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer. ACIDITY They are weak acids. The Ka value for most unsubsituted aliphatic and aromatic carboxylic acids fall within the range from 10-4 to 10-5. Recall small pKa stronger acid large pKa waker acid Ka are related as such pKa= -log(Ka) pka+pKb=14 ACIDITY Substitution of the alpha-carbon of an atom or a group of atoms of higher electronegativity than carbon increases the acidity of carboxylic acid. The inductive effect of an electronegative atom SUBSTITUENT EFFECTS ON ACIDITY 22 => SALTS OF CARBOXYLIC ACIDS Sodium hydroxide removes a proton to form the salt. Adding a strong acid, like HCl, regenerates the carboxylic acid. CH3 C OH NaOH HCl 24 O O CH3 _ + C O Na Cha => pter 20 SALTS NAMING ACID SALTS Name the cation. Then name the anion by replacing the -ic acid with -ate. - CH3CH2CHCH2COO K + potassium -chlorovalerate (common) potassium 3-chloropentanoate (IUPAC) 26 Cl COMPLETE REACTIONS AND NAME THE SALTS A) Butanoic Acid + Sodium Hydroxide B) 2-hydroxypropanoic (lactic acid) acid + sodium bicarbonate A) COOH + NaOH Butanoic Acid B) Sodium Hydroxide COONa +H2O Sodium Water Butanoate OH OH COOH + NaHCO3 2-Hydroxypropanoic Sodium Acid Bicarbonate (Lactic Acid COMMON) COONa + H2O +CO2 Sodium 2-hydroxypropanoate (sodium lactate, common) REDUCTION OF CARBOXYL GROUP TO 1 ALCOHOLS Use strong reducing agent, LiAlH4 (Lithium Aluminum Hydride) And either diethyl ether or Tetrahydrofuran (THF) as solvents; it reduces carboxylic acid to alcohol, but does not reduce it to a ketone. FISCHER ESTERIFICATION After the German Chemist Emil Fischer Carboxylic Acid (strong acid as a catalyst) + alcohol yields ester + water. All steps are reversible. Reaction reaches equilibrium. O COOH + CH3CH2OH + H COCH2CH3 + HOH ACID CHLORIDES The functional group of an acid halide is a carbonyl bonded to a halogen atom Acid chlorides are the most frequently used in lab and in industrial organic chemistry. The most common way to synthesize acid chlorides is to treat a carboxylic acid with thionyl chloride (SOCl2) or oxalyl chloride with the acid. O O C OH + O O C Cl C C Cl Cl (Oxalyl Chloride) + HCl + CO + CO2