Interactions Between Cells & the Extracellular Environment Chapter 6
advertisement
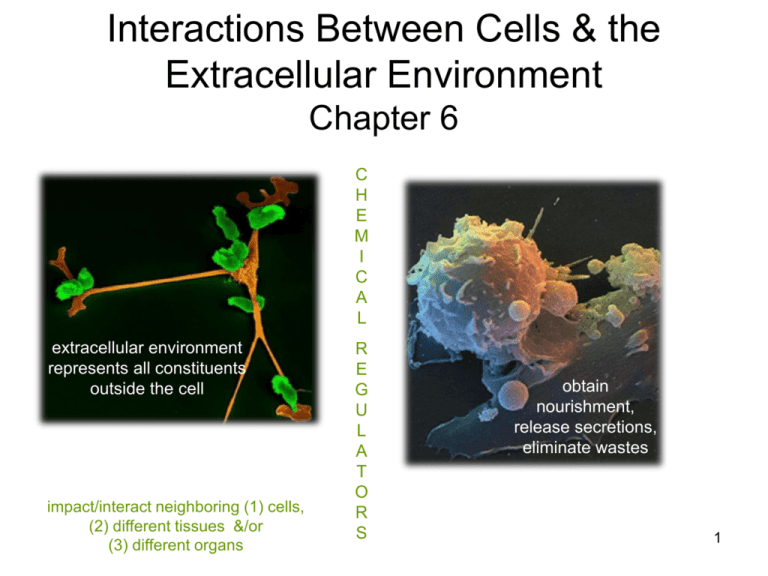
Interactions Between Cells & the Extracellular Environment Chapter 6 C H E M I C A L extracellular environment represents all constituents outside the cell impact/interact neighboring (1) cells, (2) different tissues &/or (3) different organs R E G U L A T O R S obtain nourishment, release secretions, eliminate wastes 1 Body Fluids • Divided into compartments: – extracellular • 33% • bld plasma & interstitial fluid – intracellular • 67% • fluids serve as communication link – cells tissues organs 2 Extracellular Matrix ECM • complex network of proteins – specific for any given tissue • EC fluid interspersed within • functions: – scaffolding for cellular attachment – transmits information to regulate activity, migration, growth & differentiation connective tissue of a tissue/organ 3 ECM composed of fibrous proteins but also ground substance, analogous to a hydrated gel & location of IF An Example comprised of glycoproteins & proteoglycans gel binds water highly functional, complex organization of molecules chemically linked to EC protein fibers & glycoproteins of glycocalyx block integrin - binding site on platelets, slows bld clotting integrins - adhesion molecule between cell & ECM, physically joining EC & IC compartments serve to relay signals or integrate them 4 Transport Across the Plasma Membrane • plasma membrane – serves as a “barrier” to movement - EC & IC compartments • selectively permeable – membrane transport processes • E requirements – passive transport – active transport • carrier-mediated transport versus transport without a carrier 5 Diffusion & Osmosis molecules of a solution are in constant motion solvent solute mean diffusion time conc gradient exists, motion tends to eliminate the difference, with the random motion of molecules is diffusion will move in both directions but a net movement from higher to lower until equilibrium is reached increases with distance; distances kept within 100 μm for effective exchange 6 Diffusion through a PM nonpolar molecules O2 & steroids, or small polar covalent molecules without charge CO2, ethanol & urea, can easily cross the PM will follow concentration gradient between compartments 7 Membrane Channels charged inorganic ions, Na+ & K+, utilize channels channels may be OPEN or GATED particular physiological stimuli opens/closes gate for large, polar molecules, carrier proteins are needed in the PM for movement 8 Rate of Diffusion speed of diffusion per unit time J = PA (Co – Ci) • magnitude of conc gradient • diffusing substance’s permeability to PM • temperature • SA available • distance magnitude of conc gradient driving force for diffusion BUT will not move if PM not permeable to that molecule net flow (J) is directly proportional to the conc gradient (Co-Ci), the surface area (A) and the membrane permeability coefficient (P) greater P, larger the J across the PM for any given conc difference & A 9 Osmosis • net diffusion of water – follows waters conc gradient • requirements: – conc difference of solute between sides of membrane – membrane selectively impermeable to solute • nonpenetrating solute • osmotically active – creates osmotic pressure » “pull” of water • aquaporins – water channels – facilitate movement of water – present in some cell types or can be inserted in response to regulatory molecules 10 Osmotic Pressure (OP) water drawn more rapidly with greater solute conc OP of a sol’n represents the pressure that must be applied to a solution to prevent the net flow of water indicates how strongly a sol’n draws water 11 ratio of solute to solvent not completely specified amt of water changes due to MW of substance MOLARITY Molarity vs Molality better measurement of concentration when discussing osmosis MOLALITY weigh one mole of substance and place in 1Kg water thus can compare between solutes since both in same amt of water 12 Osmolality • OP depends on ratio of solute to solvent, not chemical characteristics of solute – osmolality – Osm • total molality of a solution • what about electrolytes? – ionize • plasma & other biological fluids have a complex osmolality due to the presence of organic molecules & electrolytes – cell activity leads to constant change 13 Tonicity • describes effect of solution on osmotic movement of water, thus cell shape & volume – solute load – 300 mOsm • solutions described by how they change cell volume (& thus shape) by causing water movement • take into account both solute conc & solute permeability for each solute crossing the PM 14 Osmolality vs Tonicity Can a solution be iso-osmotic but not isotonic? YES, if solute is permeable to membrane, ie is a penetrating molecule, thus can freely cross the PM 15 Question When a cell comes in contact with a sol’n, hypertonic or hypotonic, the initial conc of solutes determines the degree of change • - impermeant If a cell were placed in a solution containing 100 mOsm impermeant solutes, how would its final volume compare to its initial volume? answer on notecard 16 Homeostasis of Plasma Concentration variety of mechanisms exist to keep blood plasma osmolality maintained within very narrow limits 17 Carrier-Mediated Transport cellular metabolism relies on the cell’s ability to uptake molecules it needs from the EC fluid many of these molecules cannot be attained by simple diffusion, require protein carriers specificity, saturation & competition Comparison carrier proteins display the characteristic of saturation if carrier can transport more one molecule type, then they will compete for transport becomes saturated 18 Facilitated Diffusion passive transport cell stimulated, insert carriers into PM to meet cell needs display specificity, competition & saturation 19 Active Transport Primary • energy required for carrier function • typically, molecules/ions moved against their conc gradient • process: – binding of molecule to be transported to “recognition site” – binding stimulates ATP hydrolysis – phosphorylation causes carrier protein to undergo conformational change – hingelike motion of carrier protein releases transported molecule to other side • often referred to as pumps 20 Na-K Pump • creates steep ion gradient • functions: – provides E for coupled transport of other molecules – used to generate electrochemical events (impulse) in nervous & muscle tissue – Na movement impt for osmotic reasons • stops, observe Nai, cause osmotic influx of water (damage cell) 21 Active Transport sets up gradient Secondary X driven indirectly by passive ion gradients created by operation of primary active pump symport what happens if the Na-K pump is poisoned? 22 Movement of solutes across a typical PM involving membrane proteins Many of these membrane proteins can be modulated by various signals, resulting in controlled rise or fall in specific solute fluxes across PM Specialized cells may contain additional transporters and channels 23 Transport Across Epithelial Membranes ♦ Since epithelial cells line the body’s surface as well as cavities of hollow organs, molecules entering the body must pass through an epithelial cell layer ♦ absorption reabsorption transcellular transport, transepithelial transport, transcytosis paracellular transport Movement of Glucose NOTICE polarity, or definite direction of transport in epithelial cells apical - basolateral 24 Junctional Complex physically join presence & number dependent on location “glued” together “velcroed” together 25 Bulk Transport endocytotic events for secretion, use exocytosis movement of molecules too large to be transport through PM 26