Photosynthesis
Ch. 8
Biology
Ms. Haut
8-1 Energy and Life
Copyright Pearson Prentice Hall
Basics of Photosynthesis
• All cells need energy to carry out their activities
• All energy ultimately comes from the sun
• Photosynthesis—process in which some of the
solar energy is captured by plants (producers) and
transformed into glucose molecules used by other
organisms (consumers).
Light energy
6CO2 + 6H2O
enzymes
C6H12O6 + 6O2
Basics of Photosynthesis
• Glucose is the main source of energy for all life.
The energy is stored in the chemical bonds.
• Cellular Respiration—process in which a cell
breaks down the glucose so that energy can be
released. This energy will enable a cell to carry
out its activities.
C6H12O6 + 6O2
enzymes
6CO2 + 6H2O + energy
Autotrophs and Heterotrophs
• Autotroph—organisms that
synthesize organic molecules
from inorganic materials (a.k.a.
producers)
– Photoautotrophs—use light
as an energy source
(plants, algae, some
prokaryotes)
• Heterotroph—organisms that
acquire organic molecules
from compounds produced by
other organisms (a.k.a.
consumers)
http://www.flatrock.org.nz/topics/animals/assets/conscious_animal.jpg
Chemical Energy and ATP
• Energy comes in
many forms including
light, heat, and
electricity.
• Energy can be stored
in chemical
compounds, too.
http://www.green-the-world.net/images/forms_of_energy.jpg
Copyright Pearson Prentice Hall
Chemical Energy and ATP
• An important chemical compound that cells use
to store and release energy is adenosine
triphosphate, abbreviated ATP.
• ATP is used by all types of cells as their basic
energy source.
Copyright Pearson Prentice Hall
http://www.accessexcellence.org/RC/VL/GG/ecb/ecb_images/03_32_ATP_and_ADP_cycle.jpg
Chemical Energy and ATP
• ATP consists of:
– adenine
– ribose (a 5-carbon
sugar)
– 3 phosphate groups
http://dm.ncl.ac.uk/helencollard/blogger/wp-content/uploads/2009/04/atp.gif
Copyright Pearson Prentice Hall
Chemical Energy and ATP
http://www.google.com/imgres?imgurl=http://student.ccbcmd.ed
u/biotutorials/energy/images/
• The three phosphate groups are the key to
ATP's ability to store and release energy.
Copyright Pearson Prentice Hall
Chemical Energy and ATP
• Storing Energy
– ADP has two phosphate groups instead of three.
– A cell can store small amounts of energy by adding a
phosphate group to ADP.
http://zymes.com/wordpress/wp-content/uploads/2007/04/atp_sr.jpg
Copyright Pearson Prentice Hall
Chemical Energy and ATP
• Releasing Energy
– Energy stored in ATP is released by breaking the
chemical bond between the second and third
phosphates.
http://zymes.com/wordpress/wp-content/uploads/2007/04/atp_sr.jpg
Copyright Pearson Prentice Hall
Chemical Energy and ATP
• The energy from ATP is needed for many
cellular activities, including active transport
across cell membranes, protein synthesis and
muscle contraction.
• ATP’s characteristics make it exceptionally
useful as the basic energy source of all cells.
Copyright Pearson Prentice Hall
Using Biochemical Energy
• Most cells have only a small amount of ATP,
because it is not a good way to store large
amounts of energy.
• Cells can regenerate ATP from ADP as needed
by using the energy in foods like glucose.
Copyright Pearson Prentice Hall
8-1
Organisms that make their own food are called
a)
b)
c)
d)
autotrophs.
heterotrophs.
decomposers.
consumers.
Copyright Pearson Prentice Hall
8-1
Most autotrophs obtain their energy from
a)
b)
c)
d)
chemicals in the environment.
sunlight.
carbon dioxide in the air.
other producers.
Copyright Pearson Prentice Hall
8-1
How is energy released from ATP?
a)
b)
c)
d)
A phosphate is added.
An adenine is added.
A phosphate is removed.
A ribose is removed.
Copyright Pearson Prentice Hall
8-1
How is it possible for most cells to function with
only a small amount of ATP?
a)
b)
c)
d)
Cells do not require ATP for energy.
ATP can be quickly regenerated from ADP and P.
Cells use very small amounts of energy.
ATP stores large amounts of energy.
Copyright Pearson Prentice Hall
8-1
Compared to the energy stored in a molecule of
glucose, ATP stores
a)
b)
c)
d)
much more energy.
much less energy.
about the same amount of energy.
more energy sometimes and less at others.
Copyright Pearson Prentice Hall
END OF SECTION
8-2 Photosynthesis: An Overview
Copyright Pearson Prentice Hall
Photosynthesis: An Overview
• The key cellular process identified with energy
production is photosynthesis.
• Photosynthesis is the process in which green
plants use the energy of sunlight to convert
water and carbon dioxide into high-energy
carbohydrates and oxygen.
Copyright Pearson Prentice Hall
The Photosynthesis Equation
• The Photosynthesis Equation
Copyright Pearson Prentice Hall
6CO2 + 6H2O
Light energy
enzymes
http://www.biologycorner.com/resources/photosynthesis.jpg
C6H12O6 + 6O2
The Photosynthesis Equation
• Photosynthesis uses the energy of sunlight to
convert water and carbon dioxide into highenergy sugars and oxygen.
Copyright Pearson Prentice Hall
A Photosynthesis Road Map
– Photosynthesis is composed of two
processes:
• The light reactions convert solar
energy to chemical energy.
• The Calvin cycle makes sugar from
carbon dioxide.
Figure 7.4
Light and Pigments
• In addition to water and carbon dioxide,
photosynthesis requires light and chlorophyll.
Copyright Pearson Prentice Hall
http://www.biologycorner.com/resources/photosynthesis.jpg
Light and Pigments
• Plants gather the sun's energy with lightabsorbing molecules called pigments.
• The main pigment in plants is chlorophyll.
• There are two main types of chlorophyll:
– chlorophyll a
– chlorophyll b
Copyright Pearson Prentice Hall
Light and Pigments
• Chlorophyll absorbs light
well in the blue-violet
and red regions of the
visible spectrum.
• Chlorophyll does not
absorb light well in the
green region of the
spectrum.
• Green light is reflected
by leaves, which is why
plants look green.
Light and Pigments
• Light is a form of energy, so any compound that
absorbs light also absorbs energy from that
light.
• When chlorophyll absorbs light, much of the
energy is transferred directly to electrons in
the chlorophyll molecule, raising the energy
levels of these electrons.
• These high-energy electrons are what make
photosynthesis work.
Copyright Pearson Prentice Hall
8-2
Plants use the sugars produced in
photosynthesis to make
a)
b)
c)
d)
oxygen.
starches.
carbon dioxide.
protein.
Copyright Pearson Prentice Hall
8-2
The raw materials required for plants to carry out
photosynthesis are
a)
b)
c)
d)
carbon dioxide and oxygen.
oxygen and sugars.
carbon dioxide and water.
oxygen and water.
Copyright Pearson Prentice Hall
8-2
The principal pigment in plants is
a)
b)
c)
d)
chloroplast.
chlorophyll.
carotene.
carbohydrate.
Copyright Pearson Prentice Hall
8-2
The colors of light that are absorbed by chlorophylls
are
a)
b)
c)
d)
green and yellow.
green, blue, and violet.
blue, violet, and red.
red and yellow.
Copyright Pearson Prentice Hall
END OF SECTION
8-3 The Reactions of
Photosynthesis
Copyright Pearson Prentice Hall
Photosynthesis: redox process
• Oxidation-reduction reaction:
– Oxidation-loss of electrons from one
substance
– Reduction-addition of electrons to another
substance
Releases e- (and H+ ions)
oxidation
Light energy
6CO2 + 6H2O
enzymes
C6H12O6 + 6O2
reduction
gains e- (and H+ ions)
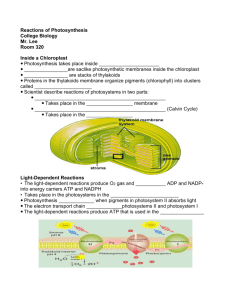
Inside a Chloroplast
• In plants, photosynthesis takes place inside
chloroplasts.
Copyright Pearson Prentice Hall
Inside a Chloroplast
• Chloroplasts contain thylakoids—saclike
photosynthetic membranes.
Copyright Pearson Prentice Hall
Inside a Chloroplast
• Thylakoids are arranged in stacks known as
grana. A singular stack is called a granum.
Copyright Pearson Prentice Hall
Inside a Chloroplast
• Proteins in the thylakoid membrane organize
chlorophyll and other pigments into clusters
called photosystems, which are the lightcollecting units of the chloroplast.
Copyright Pearson Prentice Hall
Inside a Chloroplast
• The light-dependent
reactions take place
within the thylakoid
membranes.
• The Calvin cycle
takes place in the
stroma, which is the
region outside the
thylakoid
membranes.
Copyright Pearson Prentice Hall
Electron Carriers
• Electron Carriers
– When electrons in chlorophyll absorb sunlight, the
electrons gain a great deal of energy.
– Cells use electron carriers to transport these highenergy electrons from chlorophyll to other
molecules.
• One carrier molecule is NADP+.
• Electron carriers, such as NADP+, transport electrons.
• NADP+ accepts and holds 2 high-energy electrons along with
a hydrogen ion (H+). This converts the NADP+ into NADPH.
Copyright Pearson Prentice Hall
Electron Carriers
• The conversion of NADP+ into NADPH is one way
some of the energy of sunlight can be trapped
in chemical form.
• The NADPH carries high-energy electrons to
chemical reactions elsewhere in the cell.
• These high-energy electrons are used to help
build a variety of molecules the cell needs,
including carbohydrates like glucose.
Copyright Pearson Prentice Hall
Light-Dependent Reactions
• The light-dependent reactions require light.
• The light-dependent reactions produce oxygen
gas and convert ADP and NADP+ into the energy
carriers ATP and NADPH.
Copyright Pearson Prentice Hall
1. When photosystem II absorbs light an e- is excited in the
reaction center chlorophyll (P680) and gets captured by
the primary e- acceptor.
• This leaves a hole in the P680
2. To fill the hole left in P680, an enzyme extracts e- from
water and supplies them to the reaction center
• A water molecule is split into 2 H+ ions and an
oxygen atom, which immediately combines with
another oxygen to form O2
3. Each photoexcited e- passes from primary e- acceptor to
photosystem I via an electron transport chain.
• e- are transferred to e- carriers in the chain
4. As e- cascade down the e- transport chain, energy is
released and harnessed by the thylakoid membrane to
produce ATP
• This ATP is used to make glucose during Calvin cycle
5. When e- reach the bottom of e- transport chain, it fills the
hole in the reaction center P700 of photosystem I.
• Pre-existing hole was left by former e- that was
excited
6. When photosystem I absorbs light an e- is excited in the
reaction center chlorophyll (P700) and gets captured by
the primary e- acceptor.
• e- are transferred by e- carrier to NADP+ (reduction
reaction) forming NADPH
• NADPH provides reducing power for making
glucose in Calvin cycle
Chemiosmosis
• Energy released from ETC is
used to pump H+ ions (from the
split water) from the stroma
across the thylakoid membrane
to the interior of the thylakoid.
– Creates concentration gradient
across thylakoid membrane
– Process provides energy for
chemisomostic production of
ATP
Light-Dependent Reactions
• The light-dependent reactions use water, ADP,
and NADP+.
• The light-dependent reactions produce oxygen,
ATP, and NADPH.
• These compounds provide the energy to build
energy-containing sugars from low-energy
compounds.
Copyright Pearson Prentice Hall
The Calvin Cycle
• The Calvin Cycle
– ATP and NADPH formed by the light-dependent
reactions contain an abundance of chemical energy,
but they are not stable enough to store that energy
for more than a few minutes.
– During the Calvin cycle plants use the energy that
ATP and NADPH contain to build high-energy
compounds that can be stored for a long time.
•
Copyright Pearson Prentice Hall
The Calvin Cycle
• The Calvin cycle uses
ATP and NADPH from the
light-dependent
reactions to produce
high-energy sugars.
• Because the Calvin cycle
does not require light,
these reactions are also
called the lightindependent reactions.
Copyright Pearson Prentice Hall
The Calvin Cycle
• Six carbon dioxide molecules enter the cycle
from the atmosphere and combine with six 5carbon molecules.
Copyright Pearson Prentice Hall
The Calvin Cycle
• The result is twelve 3-carbon molecules, which
are then converted into higher-energy forms.
Copyright Pearson Prentice Hall
The Calvin Cycle
• The energy for this conversion comes from ATP
and high-energy electrons from NADPH.
Copyright Pearson Prentice Hall
The Calvin Cycle
• Two of twelve 3-carbon molecules are removed
from the cycle.
Copyright Pearson Prentice Hall
The Calvin Cycle
• The molecules are used to produce sugars,
lipids, amino acids and other compounds.
The Calvin Cycle
• The 10 remaining 3-carbon molecules are
converted back into six 5-carbon molecules,
which are used to begin the next cycle.
The Calvin Cycle
• The two sets of photosynthetic reactions work
together.
– The light-dependent reactions trap sunlight energy
in chemical form.
– The light-independent reactions use that chemical
energy to produce stable, high-energy sugars from
carbon dioxide and water.
Copyright Pearson Prentice Hall
The Calvin Cycle
• Factors Affecting Photosynthesis
– Many factors affect the rate of photosynthesis,
including:
• Water
• Temperature
• Intensity of light
Copyright Pearson Prentice Hall
How Photosynthesis Moderates Global
Warming
• Photosynthesis has an enormous impact on the
atmosphere.
– It swaps O2 for CO2.
http://www.destination360.com/asia/malaysia/images/s/borneo-rainforest.jpg
How Photosynthesis Moderates Global
Warming
• Greenhouses used to grow plant indoors
– Trap sunlight that warms the air inside.
• A similar process, the greenhouse effect,
– Warms the atmosphere.
– Is caused by atmospheric CO2.
Global Warming
• Greenhouse gases (CO2,
CH4, CFC’s) are the most
likely cause of global
warming, a slow but
steady rise in the Earth’s
surface temperature.
– Destruction of forests may
be increasing this effect.
– Combustion of fossil fuels
Global Warming Consequences
• Polar ice caps melting
• Rise in sea level and
flooding of current
coastline
– New York, Miami, Los
Angeles underwater
http://i.treehugger.com/images/2007/10/24/melting%20ice-jj-002.jpg
• Change in types of
plants—more adapted to
warmer temps. and less
water
8-3
In plants, photosynthesis takes place inside the
a)
b)
c)
d)
thylakoids.
chloroplasts.
photosystems.
chlorophyll.
Copyright Pearson Prentice Hall
8-3
Energy to make ATP in the chloroplast comes most
directly from
a) hydrogen ions flowing through an enzyme in the thylakoid
membrane.
b) transfer of a phosphate from ADP.
c) electrons moving through the electron transport chain.
d) electrons transferred directly from NADPH.
Copyright Pearson Prentice Hall
8-3
NADPH is produced in light-dependent reactions and
carries energy in the form of
a)
b)
c)
d)
ATP.
high-energy electrons.
low-energy electrons.
ADP.
Copyright Pearson Prentice Hall
8-3
What is another name for the Calvin cycle?
a)
b)
c)
d)
light-dependent reactions
light-independent reactions
electron transport chain
photosynthesis
Copyright Pearson Prentice Hall
8-3
Which of the following factors does NOT directly affect
photosynthesis?
a)
b)
c)
d)
wind
water supply
temperature
light intensity
Copyright Pearson Prentice Hall
END OF SECTION