Reactivity Lab - Rothschild Science
advertisement

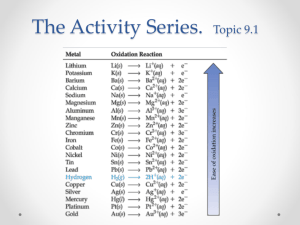
Warm Up- Separate piece of paper…NOT IN CB!! Reactivity Lab Purpose- To look at the reactivity of different metals and determine an activity series. Hypothesis Think about the number of valence electrons in an atom….Which metals do you think are most reactive? Metals with ____________ valence electrons will have _____________ reactivity, because…… How does it work? A reaction takes place when electrons move. The more reactive atom gives its electrons to the less reactive atom and “replaces” it in the compound. Na is more active than Li Na + LiCl → Li + NaCl Materials List As you do the lab, make a complete list of the materials that you are using… Procedure 1. 2. 3. Place metals in the correct well. Add the correct solution using the plastic pipettes (be careful!) Make observations for any well that seems to react. Record color changes, formation of new substances, bubbles…etc. Setting up your well plate Copy this table onto your lab 1 2 3 4 5 6 A Mg Mg Fe Mg Mg Mg B Fe Fe Zn Zn Fe Fe C Zn Cu Cu Cu Zn Zn D Pb Pb Pb Pb Cu Cu Make another table to record observations… 1 2 3 4 5 6 A B C D Adding the Solutions Column 1- Cu(NO3)2 Column 2- Zn(NO3)2 Column 3- Mg(NO3)2 Column 4- Fe(NO3)2 Column 5- Pb(NO3)2 Column 1- Ag(NO3)2 Make a table to record your observations! 1A….turned green 2B… no reaction Be very detailed in your descriptions. What did you see? Setting up your well plate Copy this table onto your lab 1 2 3 4 5 6 Mg Mg Fe Mg Mg Mg A B Fe Fe Zn Zn Fe Fe C Zn Cu Cu Cu Zn Zn D Pb Pb Pb Pb Cu Cu Here are my results A B C D 1 Y Y Y Y 2 Y N N N 3 Y Y N N 4 Y Y Y N 5 Y Y Y N 6 Y Y Y Y Valence Electrons Mg- 2 Zn- 2 Cu- 2 Fe- 2 Pb- 2 Ag- 2 When you look at the number of valence electrons, you notice that all the metals we used all the same number…. So it must have more to do with where the electrons are on the atom… Trend in Reactivity Reactivity increases down a group- the electrons are farther away from the nucleus. Look at Bohr models for Li and K Reactivity decreases from left to right across the PT because the orbitals are getting closer to being complete… Bohr model for Li and K Concluding Questions 1. 2. 3. 4. What evidence did you look for to determine if a reaction occurred? Which were the most reactive metals? Why? Which were the least reactive metals? Why? List the metals from least reactive to most reactive. Write a nice paragraph that answers all the questions. Use complete sentenses.Don’t use personal pronouns.