Chapter 8 “Molecular Shape”
Do we live in a two dimensional world?
How does molecular shape affect the
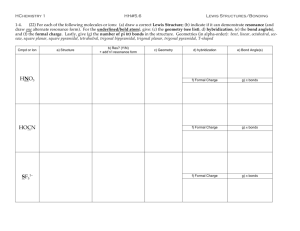
properties of compounds in our world?
T. Witherup 2006
OBJECTIVES
Identify the shapes of small molecules.
Describe and use the VSEPR theory.
Learn about hybrid orbitals.
Recognize the relationship between bond
length and bond strengths.
Explain what determines the polarity of a
molecule.
Explain why water is a polar molecule (a
dipole).
8-1 The Shape of Small Molecules
Just as architects use models to see how a building
will look when finished, chemists use models to
help visualize how atoms of molecules are
arranged in space.
Examples: Ball & stick, space filling, wire models.
The shape of a molecule (molecular geometry) is
described by the geometric figure formed when the
atomic nuclei are imagined to be joined in straight
lines.
Examples: H----Cl, H----F, C----O, O---O, O----C----O
The bond angle is the geometric angle between
two adjacent bonds.
Examples: H----Cl (180°); B----F angle in BF3 (120°)
Measuring Bond Angles
Use a protractor to determine the angle between two circles.
Place the center of the protractor on the central circle, and align
the base with a bond. Then measure the angle.
8-1 The Shape of Small Molecules
Class Activity: In groups of two, follow the
directions on “8-1 Explore” with the following
modification:
Use toothpicks in place of pipe cleaners.
The purpose of this activity is to arrange the
spheres around a central point so they are as far
apart from each other as possible.
Measure the angles carefully.
This models the way atoms are arranged around a
central atom.
Common Molecular Shapes
LINEAR (CO, HCl, CO2)
TRIGONAL PLANAR (BCl3, BF3)
TETRAHEDRAL (CH4, SiCl4)
PYRAMIDAL (NH3, PH3)
BENT (H2O, H2S)
“T”-SHAPED (ClF3)
SQUARE PLANAR (XeF4)
TRIGONAL BIPYRAMIDAL (PF5)
OCTAHEDRAL (SF6)
VSEPR Theory
The VSEPR theory helps explain the
shapes of simple molecules.
Valence Shell Electron Pair Repulsion
(VSEPR) theory states that, in small
molecules, the pairs of valence electrons
are arranged as far apart from each other as
possible (due to repulsion of electrons).
This results in geometries that minimize the
energy of molecules.
VSEPR Theory: Electron Group
Geometry & Molecular Geometry
Electron group geometry shows how
groups of electrons are arranged around a
central atom because of charge repulsion.
Molecular geometry shows how bonded
atoms are arranged around that same
central atom, again due to charge
repulsion.
Examples of VSEPR Shapes (Balloons)
(General Chemistry, Hill, Petrucci, McCreary, Perry, 4th Edition, Pearson Prentice Hall, 2005,
p389.)
2 electron groups – linear
3 electron groups – trigonal
planar
4 electron groups – tetrahedral
5 electron groups – trigonal
bipyramidal
6 electron groups – octahedral
(See textbook pages 256 -261.)
Let’s look at some examples of VSEPR Theory and structures at Georgia
Southern University:
http://cost.georgiasouthern.edu/chemistry/general/molecule/vsepr.htm
Examples of VSEPR Shapes (Molecules)
Let’s take a look as a series of examples.
Mark P. Heitz Slides (General Chemistry, 4th Ed., Hill,
Petrucci, McCreary & Perry, Chapter 10).
Slides 6 – 13.
This link allows us to compare two similar
structures:
http://www.chem.purdue.edu/gchelp/vsepr/
The following link has a full tutorial about
VSEPR Theory:
http://www.shef.ac.uk/chemistry/vsepr/
Bonding in Molecules
Remember that atomic orbital (quantum) theory was
developed because the previous atomic theories were
inadequate in explaining experimental data?
Likewise, atomic orbital theory (1s, 2p, etc.) does not
fully explain what happens to electrons in molecules, so
quantum theory, too, needed to be modified.
This may be shown by examining the water molecule.
Oxygen has six valence electrons in the 2s22p4 configuration.
This means there are vacancies in two p-orbitals, each holding
one electron that will bind with a hydrogen atom.
Since all p-orbitals are 90° apart, we would predict water to have
a right angle between its two hydrogen atoms.
EXPERIMENTALLY WATER HAS A 104.5° angle!
We saw that VSEPR theory accounts for the observed shape of
water, but it does not explain the bonding. Clearly something
else must be occurring.
Simple Bonding Orbitals in Molecules
Two s-orbitals overlap to form the covalent sigma (σ) bond of a
hydrogen molecule. (It is a single bond.)
H
+
H
forms
H-----------H
σ-bond
Simple Bonding Orbitals in Molecules
Two s-orbitals overlap to form the covalent sigma (σ) bond in a
hydrogen molecule. (It is a single bond.)
H
H
+
forms
H-----------H
Two p-orbitals may overlap in two different ways:
End-to-end, as in F2 (forms a sigma-bond, σ).
F
+
F
forms
F
F
σ-bond
σ-bond
Simple Bonding Orbitals in Molecules
Two s-orbitals overlap to form the covalent sigma (σ) bond in a
hydrogen molecule. (It is a single bond.)
H
H
+
forms
H-----------H
Two p-orbitals may overlap in two different ways:
End-to-end, as in F2 (forms a sigma-bond, σ).
F
F
+
forms
F
F
σ-bond
Side-by-side, as in ethylene C2H4 (forms a pi-bond, π).
C
+
C
forms
C======C
π-bond
σ-bond
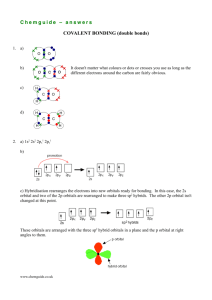
Bonding in Molecules - Hybridization
As two atoms approach to form a bond, their outer
orbitals overlap and become changed (‘perturbed’).
Hybrid orbitals are used to describe this process,
whereby the atomic orbitals mix together and have new
properties.
Hybridization of atomic orbitals means that two or more
orbitals may be mixed to form an equal number of
identical ‘hybrid orbitals.’
Consider what happens as you mix a gallon of yellow paint with
a gallon of blue paint. What is the result? Two gallons of green
paint!
We may consider orbital mixing in a similar way, although the
‘mixing’ is really a mathematical solution to complex equations!
To help us understand hybridization, we will look at some
examples.
Hybrid Orbital Example 1
CH4 is tetrahedral (by experiments), and VSEPR theory
helps explain its shape, but let’s look at this in more detail.
Carbon has this electron configuration:
C 1s2 2s2 2p2
Carbon should combine with TWO hydrogen atoms to make CH2,
not FOUR, as observed to make methane, CH4.
To explain this we may imagine that, as hydrogen atoms
approach a carbon atom, the orbitals of carbon become
mixed together in an ‘excited valence state’ of carbon.
Carbon changes to C 1s2 2s1 2p3 or four equal 2sp3 valence
orbitals, each containing only one electron, drawn as:
C
_
_
_ _ (four sp3 orbitals)
These electrons are free to pair (bond covalently in a σ-bond) with
four hydrogen atoms by sharing, becoming:
C
_
_
_ _ (full sp3 orbitals)
To be as far apart as possible from each other, the hybrid orbitals
and H atoms arrange as a tetrahedron around the carbon, as
observed experimentally for CH4.
HYBRID ORBITAL EXAMPLE 1 (cont’d)
sp3 (tetrahedral, 109.5°)
+
4
A carbon atom with a 2s22p2 electron configuration becomes an excited atom
with 2s12p3 configuration. This reacts with 4 hydrogen atoms having 1s1 electron
configurations to form four new (hybrid) orbital bonds called sp3. These are arranged
to be as far apart as possible from each other (109.4°) in the CH4 (methane) molecule.
(Means the same as
)
Reference: © Copyrght, 2001, L. Ladon. http://www.towson.edu/~ladon/carbon.html
HYBRID ORBITAL EXAMPLE 2
sp2 (trigonal planar, 120°)
In a similar way a carbon atom with a 2s22p2 electron configuration becomes
an excited atom that reacts with two hydrogen atoms having 1s1 electron
configurations to form three new (hybrid) orbital bonds called sp2. These
also are arranged to be as far apart as possible from each other (120°). The
remaining p orbital becomes part of a double bond. (The molecule ethylene,
C2H4, has this type of hybrid orbitals where the two carbons are bonded
together by one of the three sp2 (sigma) and a p-orbital (pi) bond.)
H2C=CH2
Reference: © Copyrght, 2001, L. Ladon. http://www.towson.edu/~ladon/carbon.html
HYBRID ORBITAL EXAMPLE 3
sp (linear, 180°)
Also, a carbon atom with a 2s22p2 electron configuration becomes an excited
atom that may react with a hydrogen atom having 1s1 electron configuration to
form two new (hybrid) orbital bonds called sp. These also are arranged to be as
far apart as possible from each other (180°). The remaining 2p orbitals become
part of a triple bond. (The molecule acetylene, C2H2, has this type of hybrid
orbitals where the two carbons are bonded together by a one sp (sigma) and
two p-orbitals (pi).)
HCΞCH
Reference: © Copyrght, 2001, L. Ladon. http://www.towson.edu/~ladon/carbon.html
HYBRID ORBITALS of CARBON
sp3 (tetrahedral, 109.5°) (CH4)
All single bonds (sigma, σ); end-to-end overlap.
sp2 (trigonal planar, 120°) (H2C=CH2)
Single bond skeleton (sigma, σ) (H-C & C-C).
Double bond forms from the remaining p-orbital of
carbon to form a pi bond (π); sideways overlap
above & below the skeleton.
sp (linear, 180°) (H-CΞC-H)
Single bond skeleton (sigma, σ) (H-C-C-H).
Triple bond forms from the remaining two p-orbitals
to form two pi bonds; sideways overlap is
perpendicular to each other.
HYBRID ORBITAL FACTS
ATOMIC
ORBITALS
USED BY
CENTRAL
ATOM
HYBRIDIZATION @
CENTRAL
ATOM
NUMBER
OF
HYBRID
ORBITALS
SHAPE OF
HYBRID
ORBITALS
EXAMPLE
UNUSED
ORBITALS
s, p
sp
2
Linear (180°)
BeCl2
Two p
orbitals
s, p, p
sp2
3
Trigonal
Planar (120°)
BF3
One p orbital
s, p, p, p
sp3
4
Tetrahedral
(109.5°)
CH4
None
s, p, p, p, d
sp3d
5
Trigonal Bipyramidal
(120° & 90°)
PCl5
Varies
s, p, p, p, d,
d
sp3d2
6
Octahedral
(90° & 90°)
SF6
Varies
Examples of Molecules with Hybrid
Orbitals (Let’s draw Lewis structures of these.)
Ethane, C2H6
Benzene, C6H6
Formaldehyde, H2CO
Acetic acid, H3CCOOH
Propyne, H3CCCH
WHAT ABOUT THE BONDS?
BOND
AVG. BOND LENGTH
AVG. BOND
(nm)
STRENGTH (kJ/mol)
H–H
0.075
436
F–F
0.128
155
Cl–Cl
0.198
242
Br–Br
0.228
193
I–I
0.266
151
O=O
0.121
498
C–C
0.154
346
C=C
0.134
610
CΞC
0.120
835
What trends do you notice? How can we explain them?
PROPERTIES OF BONDS
In our models the bonds are all the same length,
but this is NOT true in reality.
As one moves down a Group of the Periodic Table,
the atoms form longer bonds.
Atoms get larger moving down a Group.
Multiple bonds are shorter and stronger than single
bonds.
The more electrons in a bond, the greater the
attraction to the positive nuclei of a bond.
Electrons act as the ‘electrical glue’ between the two
nuclei.
Let’s look at some bond lengths in more detail.
Look for the trends, and explain them.
Characteristics of Various Bonds)
BOND TYPE
DISTANCE (nm)
STRENGTH (kJ/mol)
H-H
Single
0.075
436
C-H
Single
0.109
413
F-F
Single
0.128
155
Cl-Cl
Single
0.198
242
Br-Br
Single
0.228
193
I-I
Single
0.266
151
O-O
Single
0.132
146
O=O
Double
0.121
498
S=S
Double
0.189
C-O
Single
0.143
358
C=O
Double
0.121
745
CΞO
Triple
0.113
1046
C-N
Single
0.147
305
C=N
Double
0.138
615
CΞN
Triple
0.116
887
C-C
Single
0.154
347
C=C
Double
0.134
614
CΞC
Triple
0.120
839
BOND
(Source: Textbook and Teaching resources; also Kotz & Treichel, “Chemistry & Chemical Reactivity,”
5th Ed., Thomson, Brooks/Cole, 2003, pp 354-356.)
8-2 POLARITY
Recall what we learned about polar & non-polar bonds.
Are electrons shared equally in all bonds?
What happens if they are not shared equally?
See this link to find out:
http://cost.georgiasouthern.edu/chemistry/general/molecule
/polar.htm
Because of the polarity of bonds and how they are
arranged around a central atom, molecules may also be
polar or non-polar.
Dipole: a polar molecule, one that has a positive end
and a negative end.
What determines the polarity of a molecule?
The shape of a molecule and the polarity of its
bonds together determine if a molecule is polar
or non-polar.
Let’s look at some examples.
POLARITY: EXAMPLES
Hydrogen Chloride:
HCl
CO
Carbon Monoxide:
Carbon Dioxide:
OCO
Formaldehyde:
H2CO
Water:
HOH
Arrows show the direction of
negative charge in a bond,
toward the most
electronegative atom.
Use δ+ and δ– to indicate
the charges.
Is water linear? Why or why not?
Proteins: See http://jmol.sourceforge.net/
(Go to the Jmol Demonstration Pages.)
Properties of Polar Molecules
Polar molecules align in an electric field.
Similar to a compass aligning with Magnetic North.
Formaldehyde
A dipole because of the imbalance of polar bonds.
A gas, but very soluble in water.
Carbon Dioxide
Not polar because the effect of the two polar bonds cancel.
Gas at room temperature due to lack of attraction between
molecules; also soluble in water.
Water
A dipole because the bent shape of the molecule does not
cancel the polar bonds.
The shape of the water molecule has a major impact on it’s
properties, such as melting, boiling points, solubility and physical
states at room temperature.
Large Molecules
Dipoles are critical to the functions of life.
Proteins, DNA/RNA, carbohydrates, lipids, etc.
Did we cover the OBJECTIVES?
Describe and use the VSEPR theory.
Identify the shapes of small molecules.
Linear, trigonal planar, tetrahedral, pyramidal, bent, Tshaped, square planar, trigonal bipyramidal, octahedral.
Learn about hybrid orbitals.
Recognize the relationship between bond length
and bond strengths.
Explain what determines the polarity of a
molecule.
Explain why water is a polar molecule (a dipole).