Alkynes
advertisement
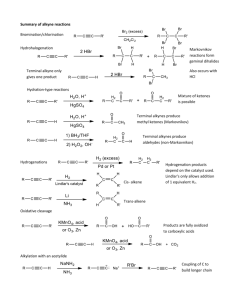
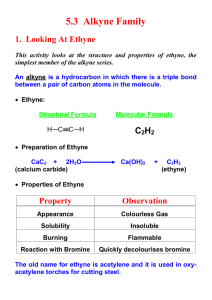
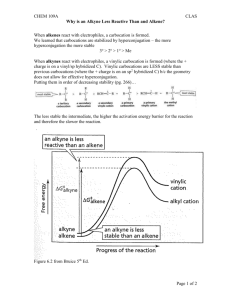
TOPIC 4: ALKYNES: STRUCTURE, REACTIVITY AND SYNTHESIS Alkynes are hydrocarbons that contain a carbon-carbon triple bond, which is the strongest and shortest type of carbon-carbon bond that exists. Many of the reactions of alkynes are similar in nature to those of alkenes, but there are important differences too. In this unit, we will examine the structure and reactivity of alkynes as well as how many of the reactions you have learned about thus far are used in the important process of organic synthesis. I. NAMING ALKYNES * Alkynes follow the general set of naming rules we saw for alkanes and alkenes, with the name of the parent chain ending in –yne. 1. Find the parent hydrocarbon. Find the longest carbon chain that contains the triple bond. Name accordingly using –yne as the suffix. 2. Number the carbon atoms in the chain. Begin at the end nearest the triple bond. If the triple bond is equidistant from the two end points, begin at the end nearest the first branch point. 3. Write the full name. • Number the substituents according to their positions on the chain, and arrange alphabetically, just as for alkanes. • Indicate the position of the triple bond by giving the number of the first carbon where it begins. • If more than one triple bond exists, use numbers to indicate the position of each and use the suffixes –diyne, -triyne, etc. • Compounds containing both double and triple bonds are called enynes. Numbering of an enyne chain begins nearer the first multiple bond, whether double or triple. A number is used to indicate the positions of both the double and triple bond. Where a tie exists, the double bond receives the lower number. 7 2 1 8 4 3 5 6 5-ethyl-4-methyl-1-octen-6-yne II. PREPARATION OF ALKYNES: ELIMINATION REACTIONS * Alkynes can be prepared by elimination of X2 from a 1,2dihalide by treatment with excess strong base or by elimination of HX from a vinylic halide by treatment with strong base. ELIMINATION OF X2 FROM 1,2-DIHALIDES H Br 2 KOH C C C Br C H + 2 H2O + 2 KBr ELIMINATION OF HX FROM VINYLIC HALIDES VINYLIC: - Refers to a substituent directly attached to a double bond H NaNH2 C C C C Br + NH3 + NaCl III. REACTIONS OF ALKYNES: ADDITION OF HX AND X2 Based on electronic similarity between alkynes and alkenes, you may expect the chemical reactivity of the two functional groups to be similar. While there are indeed many similarities, significant differences also exist. ELECTROPHILIC ADDITION OF HX - Reaction can be stopped after addition of 1 equivalent of HX - Follows Markovnikov’s Rule (X adds to more sub. carbon) - trans stereochemistry of H and X typically occurs - Addition of excess HX forms the dihalide CH3C ≡ CH HBr Br HBr CH3C = CH H add’n of 1 eq. of HBr Br H CH3C ─ C─H Br H add’n of excess HBr yields dihalide ELECTROPHILIC ADDITION OF X2 - Reaction can be stopped after addition of 1 equivalent of X2 to give the di-substituted product - Excess addition of X2 yields the tetra-substituted product - Trans stereochemistry results CH3CH2C ≡ CH Br2 CH3CH2 Br C=C Br H Br2 CH3CH2CBr2CHBr2 Reaction Mechanism: The reaction mechanism of electrophilic addition of HX to an alkyne is similar to that of an alkene. It occurs in two steps with a vinylic carbocation intermediate forming. H Br H RC CH Br H R C C H Br C R C H Vinylic carbocation intermediate * Although the mechanism for the addition of HX to an alkyne mirrors that of the mechanism for the addition of HX to an alkene, most other alkyne additions occur through more complex mechanistic pathways. IV. HYDRATION OF ALKYNES: ADDITION OF H2O * Like alkenes, alkynes can be hydration via two different methods. Addition of H2O in the presence of acid yields the Markovnikov product while indirect addition of H2O via hydroboration yields the anti-Markovnikov product. Unlike in the case of alkenes however, the product of hydration is not exactly an alcohol. This is due to the existence of what is known as tautomerisation. H O Rapid O C C C H C Enol tautomer Keto tautomer (less favored) (more favored) ENOL: - a vinylic alcohol (ene + ol) (-OH is directly attached to a double bonded carbon) H O - less stable than its keto counterpart C C TAUTOMERS: - describes constitutional isomers that interconvert rapidly - in keto-enol tautomerism, the keto tautomer is favored (*see example above) *Note: tautomerisation is not the same as resonance. In resonance, atoms do not move, only electrons do. In tautomerisation, atoms switch places. Also, resonance is a concept of how molecules exist: they do not actually switch back and forth between resonance structures. In tautomerisation, molecules actually do shift back and forth between isomeric structures. A. Acid- Catalyzed Hydration of Alkynes: Markovnikov Product - Reaction of an alkyne w/ H2O in the presence of acid to form an enol at more substituted carbon (Markonikov product) - Enol undergoes tautomerisation to give the favored ketone product * See board for mechanism Reaction Mechanism: H H CH3C CH H H H3C C H2O C O + C H more substituted vinyllic carbocation forms H3C H H2O C H H HO C C H3 C H tautomerisation O C H3 C * CH3 *Note: This reaction is most useful when applied to a terminal alkyne because only one product forms. When the reaction occurs with an internal alkyne, a mixture of ketone products form. O O R C C R' H2O C R H2SO4 CH2R' an internal alkyne + C RCH2 mixture O R C C H H2O H2SO4 an external alkyne C R CH3 single product R' B. Hydroboration of Alkynes: anti- Markovnikov Product - Reaction of an alkyne w/ BH3 in the presence of H2O2/ OH─ to form an enol at less substituted carbon (Markonikov product) - Enol undergoes tautomerisation to give the favored keto product * See board for mechanism *Again, this reaction is most useful when applied to a terminal alkyne because only one product, in this case an aldehyde, forms. When the reaction occurs with an internal alkyne, a mixture of products form. O R C C H 1. BH3 2. OH- / an external alkyne H2O2 C R H single aldehyde product Reaction Mechanism: CH3C CH 1. BH3 BH2 H C H3 C BH2 H C C H Transition State: BH3 adds to less substituted carbon H3 C 2. H2O2 C OH- OH replaces BH2 H OH H C C * H3C H tautomerisation O H3C C CH2 * H V. REDUCTION OF ALKYNES * Alkynes are easily reduced, which means that they form an increase in bonds to hydrogen, by addition of H2 over a metal catalyst. As a result, alkynes can be reduced to an alkene or further to an alkane. Depending on what reagents are used in the reduction, either product can be selected for. HC CH reduction H2C CH2 reduction H3C CH3 Ethyne Ethene Ethane alkyne alkene alkane A. Complete Reduction: Alkyne to Alkane - Reduction to the alkane occurs when the alkyne is reacted w/ H2 in the presence of palladium on carbon (Pd/C) as a catalyst H2 Pd/C alkyne alkane B. Incomplete Reduction: Alkyne to Alkene PATH 1: Formation of Cis Alkenes - Incomplete reduction to the alkene occurs when the alkyne is reacted with H2 in the presence the less active Lindlar catalyst - The use of H2/ Lindlar catalyst produces CIS alkenes. H H H2 Lindlar alkyne catalyst cis alkene PATH 2: Formation of Trans Alkenes - Incomplete reduction to the alkene also occurs when the alkyne is reacted w/ Na or Li metal in liquid ammonia (NH3). - The use of Na or Li/ NH3 produces TRANS alkenes. H Li NH3 alkyne H trans alkene VI. OXIDATIVE CLEAVAGE OF ALKYNES * Alkynes, like alkenes, can by cleaved by reaction with a powerful oxidizing agent like ozone. A triple bond is generally less reactive that a double bond however, and yields of cleavage products are sometimes low. Like alkenes, the products of alkyne cleavage produce carbonyls, but as part of a different functional group. Oxidative Cleavage of Alkynes: - The products of cleavage of an internal alkyne (R─C≡C─R’) are two carboxylic acids - The products of cleavage of an external alkyne (R─C≡C─H) are a carboxylic acid and CO2 O O3 1 2 + Zn/H3O O + 1 OH HO 2 internal alkyne H 1 2 external alkyne O3 Zn/H3O+ O 1 OH + CO2 2 VII. ALKYNE ACIDITY: FORMATION OF ACETYLIDE ANIONS * According to the Brønsted- Lowry definition, an acid is any substance that donates H+. Although we usually think of oxyacids (H2SO4, H3PO4) or halogen acids (HCl, HBr) in this context, any compound containing a hydrogen atom can be an acid under the right circumstances. The most striking difference between alkynes and alkenes and alkanes is that terminal alkynes are weakly acidic. Acidity of Simple Hydrocarbons Type Example Ka pKa Alkyne HC ≡ CH 10─25 25 Alkene H2C= CH2 10─44 44 Alkane H3C− CH3 10─60 60 Stronger acid Weaker acid When a terminal alkyne is treated with a strong base, such as sodium amide (Na+NH2─), the terminal hydrogen is removed and an ACETYLIDE ANION is formed: R C C external alkyne H NH2 Na R C C Na NH3 Acetylide anion The presence of a negative charge and an unshared electron pair nucleophilic on carbon makes an acetylide anion extremely ________________. ALKYLATION REACTION: - Substitution reaction in which a new alkyl group becomes attached to the starting alkyne - The nucleophilic acetylide anion attacks a positively polarized carbon atom in a haloalkane - This a BIG deal a new carbon – carbon bond is formed! H H R C C Na H C Br H R C C C H H NaBr The reaction conditions for acetylide anion formation and alkylation are often shown together as a two step reaction process. acetylide anion formation CH3CH2CH2C CH 1. NaNH2 2. CH3CH2Br alkylation CH3CH2CH2C CCH2CH3 - Acetylide anion alkylation is limited to primary alkyl bromides and iodides. - In addition to their reactivity as nucleophiles, acetylide anions are sufficiently strong bases that they can cause dehydrohalogenation (loss of H + halogen) instead of substitution when they react with secondary and tertiary alkyl halides. R H + NaBr H substitution product (acetylide anion undergoes alkylation) H Br H R C C Na H H H + HBr o 2 alkyl bromide H elimination product (acetylide anion acts as a base)