23.2 Step by Step for Balancing
advertisement
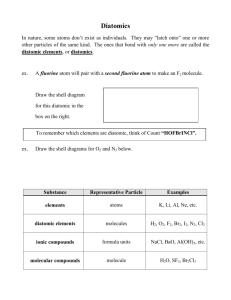
Balancing Chemical Equations 1) TEKS: 8.5F recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of mass. Prior Knowledge: Students should be able to define atoms, elements, molecules, and compounds before beginning this assignment. They should also have a general understanding of the periodic table of elements. Lesson objectives Teachers' notes Teacher's notes con't Subject: Chemistry Grade(s): Topic: Chemical Equations Cross-Curricular Links: 8 . Lesson notes: Intended Learning Outcome: This lesson is an introduction to balancing chemical equations. This assignment helps show how compounds are a unit and can be added only as groups. Instructions: Each slide contains a chemical equation. Students are to balance the equation by cloning the graphic element boxes so that there are equal amounts of all atoms on both sides. When this is accomplished, the coefficients should be written in the equation. Slide 5 contains a video tutorial on balancing equations. The video solves the same chemical equation shown on that slide. Lesson Objectives Teacher's notes Teacher's notes con't Lesson notes continued: __H The compounds/molecules are already created on each slide; however, the teacher could choose to build them with his or her students to represent how the atoms are grouped. Edited by: Lisa Jackson, ITS, NEISD Authored by: Bret Gensburg, University of Akron Lesson Objectives Teacher's notes Teacher's notes con't Pull here for molecule and equation samples Optional Enhancement: EXAMPLE: Balance the following equation. A balanced equation has the same number of atoms of each element on both sides of the equation. Clone the molecules which contain atoms of which you do not have enough. Then count the molecules and write the coefficient in the chemical equation. __H2 +__O2 __H2O Video Tutorial Work through this sample together, or click on the video file below to watch, listen, and learn how to balance a chemical equation. balancing chemical equations.avi Answer 2H2 + O2 2H2O Balance the following equation so that there are the same number of atoms on each side of the yield sign. (Clone the molecules as needed.) Click on the answer box to reveal the answer. What numbers should be added to the equation, if any? Write them in the spaces below. __CaCO3 __CaO + __CO2 Answer CaCO3 CaO + CO2 Balance the following equation. __C + __Cl2 __CCl4 Answer C + 2Cl2 CCl4 Balance the following equation. __H2 + __Cl2 __HCl Answer H2 + Cl2 2HCl Balance the following equation. __Na + __F2 __NaF Answer 2Na + F2 2NaF Balance the following equation. __H2O2 __H2O + __O2 Answer 2H2O2 2H2O + O2 Balance the following equation. __KClO3 __KCl + __O2 Answer 2KClO3 2KCl + 3O2 Balance the following equation. __P + __O2 __P4O10 Answer 4P + 5O2 P4O10 Balance the following equation. __BaCl2 + __H2SO4 __BaSO4 + __HCl Answer BaCl2 + H2SO4 BaSO4 + 2HCl Balance the following equation. __N2 + __H2 __NH3 Answer N2 + 3H2 2NH3 Balance the following equation. __Na + __Cl2 __NaCl Answer 2Na + Cl2 2NaCl Balance the following equation. __Fe + __O2 __Fe2O3 Answer 3Fe + O2 Fe2O3