Chapter 9 Chemical Equations
advertisement

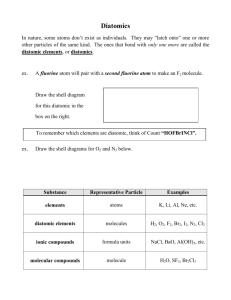
CHAPTER 9 CHEMICAL EQUATIONS CHEMICAL CHANGES AND HOW CHEMICAL REACTIONS ARE WRITTEN WHAT IS A CHEMICAL REACTION? Chemical reactions are CHEMICAL CHANGES. Evidence of a chemical change: • Light • Heat • Formation of a gas • change in color • Formation of a precipitate **Particles must collide for a chemical reaction to occur WHAT IS A CHEMICAL REACTION? Physical change: when only the physical structure of the material has changed. Chemical change: where the chemical structure of the material AND physical structure of the material has changed. REACTIONS INVOLVE REARRANGEMENTS OF ATOMS Law of conservation of mass states: mass cannot be created or destroyed! Start and end with the same number of atoms, just in a different arrangement In a sense its like musical chairs, but with atoms. CHEMICAL REACTIONS RELEASE OR ABSORB ENERGY Exothermic Reactions: heat is released Endothermic Reactions: heat is absorbed HOW ARE CHEMICAL EQUATIONS FOR REACTIONS WRITTEN? A word equation describes a reaction and translates into a formula equation. Ethanol reacts with oxygen to produce carbon dioxide and water. Word equation: Ethanol + oxygen carbon dioxide + water Formula equation: CH2CH3OH + O2 CO2 + H2O KEY WORDS TO LOOK FOR!! • • • • Reacts with = + Forms, produces = Combustion = + O2 Any HONCLBrIF element stated is DIATOMIC!!! PRACTICING WRITING CHEMICAL EQUATIONS 1. Iron reacts with oxygen to form iron (III) oxide and releases 824.8 kJ of energy 1. Heat is added to dinitrogen pentoxide to produce dinitrogen tetroxide and oxygen gas. BALANCING CHEMICAL EQUATIONS BALANCING CHEMICAL EQUATIONS Coefficients: show how much of each compound (either reactants or products) is present. Subscripts: show the number of each type of atom in a formula Superscripts: tell the type of charge on an ion 2CH3 SO42- GENERAL GUIDELINES FOR BALANCING CHEMICAL REACTIONS 1. Balance elements that are found in more than one reactant or product LAST!! 2. If the same polyatomic ions appear on both sides of the equation, treat them as a single unit 3. Balance elements, starting with the reactants BALANCING CHEMICAL REACTIONS PRACTICE Example 1The first step in the commercial production of nitric acid is the combustion of ammonia in the presence of a catalyst. The exothermic reaction produces nitrogen monoxide and water vapor. Write and balance the equation for the reaction. ANSWER • Word equation: Ammonia + oxygen nitrogen monoxide + water +heat • Unbalanced formula equation: __NH3 + __O2 __NO + __H2O + heat • Balanced formula: 4NH3 + 5O2 4NO + 6H2O + heat BALANCING CHEMICAL REACTIONS PRACTICE Example 2- Hydrogen reacts with chlorine to produce hydrochloric acid (hydrogen chloride). What is the balanced chemical equation? • Word equation: Hydrogen + chlorine hydrogen chloride • Unbalanced formula equation: __H2 + __Cl2 __HCl • Balanced formula: H2 + Cl2 2HCl HOMEWORK Page 316- #1 Page 317- #’s 6, 7, 8, 9 DUE NEXT TIME!!