Balancing Chemical Equations Practice Worksheet
advertisement

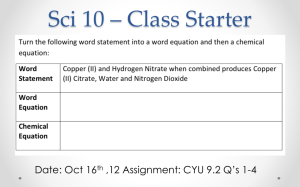
Balancing Chemical Equations Practice/Review Name: Blk:___ Please identify the SUBSCRIPT ( # of atoms) within each compound below 1) CO2 2) H2O2 3) NaFCl3 4) Mg4O2 Please identify the COEFFICIENT of each compound below AND the # of atoms within each compound 1) 3NaCl2 2) 5LiFAg2 3) HeO3 4) 2Cu2N3 Please complete the following chemical equations. PLEASE SHOW ALL WORK!! Then IDENTIFY the REACTANTS & PRODUCTS in each of the chemical reactions 1) 68 g of iron reacts with 150 g of chlorine to produce ____________g of iron chloride. Reactant/s: ____________________ Product/s: _____________________ 2) 52 g of hydrogen reacts with ________ g of Oxygen to produce 110 g of hydrogen peroxide. Reactant/s: ____________________ Product/s: _____________________ 3) _______ g of strontium reacts with 133 g of bromine to produce 184 g of strontium bromide. Reactant/s: ____________________ Product/s: _____________________ 4) Chemical A has a mass of 10 grams, and Chemical B has a mass of 16 grams. If Chemical A & Chemical B combine to create a chemical reaction, what will be the mass of the product?__________ 5) The chemical formula for water is H2O, which means there are ____ H atoms for every ____ O atom. If you have 4 molecules of H2O (4H2O), how many individual atoms of Hydrogen and Oxygen do you have? ______ H _______ O 5) 10 atoms of Nitrogen and 20 atoms of Oxygen go through a chemical reaction, how many atoms of Nitrogen and Oxygen are present as the product at the end of this chemical reaction? _____________ …..YES…there IS a BACK….PLEASE TURN PAPER OVER>>>>>>> NOW!! Balancing Chemical Equations Rules: 1) You CAN NOT change the ___________! Can ONLY change the ________________ 2) It does NOT have to _________ the same on BOTH sides, it only has to be ______________! 3) You CAN NOT split COMPOUNDS to put in a ________________! 4) You can ONLY MULTIPLY through when it is a _______________, because they are already _______ 5) CAPITAL letters are each a ________________ ______________. A CAPITAL letter followed by a lower case letter is all _________ __________________. Balancing Chemical Equations: 1) Separate the _________________ from the _____________________ by drawing a line. 2) Write down the CHEMICAL SYMBOL of each of the __________________ on each side of the equation. 3) Using the _____________ and __________________write down the number of each element. 4) If the _______________ and the __________________ are NOT balanced, change ONLY the _______________ by using trial and error. You will have to go back and forth between each side. Always ask yourself, “What is the smallest # that I can multiply the lower # by to equal it out?”