PPT: 4.4 Intermolecular Forces
advertisement

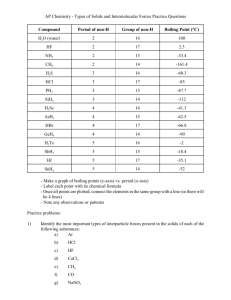
TOPIC 4: CHEMICAL BONDING & STRUCTURE ESSENTIAL IDEA The physical properties of molecular substances result from different types of forces between their molecules. NATURE OF SCIENCE (2.2) Obtain evidence for scientific theories by making and testing predictions based on them – London dispersion forces and hydrogen bonding can be used to explain special interactions. For example, molecular covalent compounds can exist in the liquid and solid state. To explain this, there must be attractive forces between their particles which are significantly greater than those that could be attributed to gravity. INTRAMOLECULAR FORCES Bonding forces that exist within each molecule. Affect molecular geometries, physical properties and reactivity. INTERMOLECULAR FORCES Non-bonding force that exist between the molecules. The strength of the intermolecular forces influence physical properties such as volatility, melting and boiling points The stronger the forces, the higher the melting and boiling points. Intermolecular forces are much weaker than intramolecular forces! UNDERSTANDING/KEY IDEA 4.4.A Intermolecular forces include London dispersion forces, dipoledipole forces and hydrogen bonding. TYPES OF INTERMOLECULAR FORCES London Forces (Dispersion Forces) Dipole-dipole Forces Hydrogen bonds Van der Waals Forces – include London and dipole- dipole forces LONDON DISPERSION FORCES These are the weakest of the intermolecular forces. Occurs between ALL molecules, polar and non-polar Substances held together with London dispersion forces have low melting and boiling points and are often gases at room temperatures. These forces occur when electron clouds shift to form temporary dipoles which then induce dipoles in neighboring molecules so that they can attract each other. The larger the electron cloud, the stronger the London dispersion forces because there is a higher probability of temporary dipoles forming. LONDON (DISPERSION) FORCES www.studybite.com LONDON DISPERSION FORCES Why does the temporary dipole occur? Electrons move randomly within the electron cloud When electrons move to one region of the cloud they may form a temporary dipole where one region of that atom/molecule has a area of negative charge and the end having a positive charge The temporary dipole will cause the electrons in another molecule to be repelled (or move away). This will cause an induced dipole (since the electrons moved away, that end of the molecule is more +, while the other end becomes more -) The net result is that there is on average a stronger attractive force between molecules than repulsive. LONDON FORCES London dispersion forces are responsible for the fact that non-polar molecules can be condensed to form liquids and sometimes solids. London dispersion forces are also present in polar molecules but are often overlooked because they are so much weaker than dipole-dipole forces. umdberg.pbworks.com FACTORS AFFECTING STRENGTH OF LONDON FORCE Number of electrons How will more electrons affect polarizability? More electrons lower electrostatic attraction between valence e- and nucleus Easier to polarize stronger London force Stronger intermolecular forces meaning higher boiling points Size (volume) of Electron Cloud Larger e- cloud ( molecule ∴ molecular mass) Less attraction of e- to nucleus Easier to polarize stronger London force Larger e- cloud (molecular mass) = higher boiling pts FACTORS AFFECTING STRENGTH OF LONDON FORCE Shape of Molecules How will the shape of a molecule affect it’s interactions with other molecules? • More linear easier to access surface so more interactions • More clustered molecules (balllike) allows less surface area = less interactions between molecules • More interactions between molecules = higher boiling points http://www.slideshare.net/Hoshi94/states-of-matter-11767927 DIPOLE-DIPOLE FORCES These are stronger than London dispersion forces and the strength depends upon the degree of polarity. These intermolecular forces are caused when molecules with permanent dipoles (areas of pos/neg charge) attract each other. The stronger the dipole attraction – the higher the boiling point. DIPOLE-DIPOLE FORCES Dipole = polar molecule Dipoles will change their direction so that their oppositely charged ends are near to one another. The electrostatic attraction between the ends is dipoledipole force Dipole-dipole force HYDROGEN BONDING This is the strongest of the intermolecular forces . Hydrogen bonding is a type of dipole-dipole attraction. Hydrogen bonds form when hydrogen bonds to either nitrogen, fluorine or oxygen. The strength of the hydrogen bond is due to the small size of hydrogen and the large electronegativity of N, O and F. Water is a unique substance with hydrogen bonding in that the solid is less dense than the liquid. HYDROGEN BONDING HYDROGEN BONDING Hydrogen bonding results between the interaction of the lone pair of one atom (small and highly electronegative, like N, O or F) with the hydrogen atom of another molecule The molecule involved with hydrogen bonding should have at least one lone pair. More lone pairs= stronger hydrogen bond The more electronegative the atom the stronger the hydrogen bond Part way between a dipole-dipole bond and a dative covalent bond H- bonds have large effect on properties and structure of materials UNDERSTANDING/KEY IDEA 4.4.B The relative strengths of these interactions are London dispersion forces < dipole-dipole forces < hydrogen bonds. Intermolecular Forces Summary IM Force General Description Relative Strength (kJ/mol) Effect on BP London All molecules, Temporary dipoles from uneven edispersion Weak 1-10 Increases with # e, volume of ecloud, Shape ( S.A) DipoleDipole Electrostatic attraction between dipoles Weak to Moderate 3-25 Increases with larger polarity (E.N. diff.) Hydrogen Bond H bonded to N,O, or F creating high dipole moment. H+ attracted to lone pairs of another molecule Moderate to Strong (10-40) Increases with more lone pairs APPLICATION/SKILLS Be able to deduce the types of intermolecular forces present in substances, based on their structure and chemical formula. GUIDANCE The term “London dispersion forces” refers to instantaneous induced dipoleinduced dipole forces that exist between any atoms or groups of atoms and should be used for non-polar entities. The term “van der Waals forces” is an inclusive term, which includes dipole-dipole, dipoleinduced dipole and London dispersion forces. Intermolecular Forces All molecules will have some type of van der Waal’s force. Non-polar molecules have only London dispersion forces. Polar molecules have dipole-dipole forces and London dispersion forces. Hydrogen bonding exists when the positive hydrogen bonds with lone pairs of electrons on nitrogen, oxygen and fluorine. Van der Waals’ forces The umbrella term “van der Waals’ forces is used to include both London dispersion forces and dipoledipole attractions. It also covers the less common type of attraction known as the dipole-induced dipole. It refers to all forces between molecules that do not involve electrostatic attractions between ions or bond formation. London Dispersion Force: Cl2 --- Cl2 Dipole – Dipole attraction: HCl --- HCl Dipole-induced dipole: HCl --- Cl2 APPLICATION/SKILLS Be able to explain the physical properties of covalent compounds (volatility, electrical conductivity, and solubility) in terms of their structure and intermolecular forces. Using Forces to Predict Properties Volatility, solubility and conductivity can all be predicted and explained from knowledge of the nature of the forces between molecules. VOLATILITY How easily a substance evaporates (becomes a gas) The weaker the intermolecular force, the higher the volatility (easier for atoms to move apart) London dispersion > dipole-dipole > hydrogen bonding Ionic compounds and giant covalent compounds have low volatility. Ionic, giant covalent< polar covalent < non polar ELECTRICAL CONDUCTIVITY Covalent compounds do not contain ions; therefore, they do not conduct electricity in the solid or liquid state. Some polar covalent compounds, such as HCl which can ionize in water, will conduct electricity. Ionic compounds conduct electricity in the molten or aqueous state. Giant covalent structures are generally non-conductors except for graphite, graphene, fullerene and Si. SOLUBILITY Non-polar compounds dissolve in non-polar solvents. Polar compounds dissolve in polar solvents. Solubility is reduced in larger molecules where the polar bond is only part of the total structure. Ionic compounds are very soluble in water and nonsoluble in non-polar solvents. Giant covalent structures are non-soluble in both polar and non-polar solvents due to the very strong covalent bonds within their structure. BOILING/MELTING POINT COMPARISON The stronger the forces between atoms/molecules, the more energy required to break atoms/molecules apart, the higher the boiling and melting points. It is easiest to compare boiling points for similar substances. To predict boiling points, you must consider the type of intermolecular forces involved. To identify the force: Draw Lewis structures Determine if molecule is polar or non-polar If polar, determine if there are any H-bonds BOILING/MELTING POINT COMPARISON After the forces are identified you: Compare the size of the molecule (Mr) for London Forces For Dipole-dipole look at difference in EN of atoms If H-bonds, how many lone pair electrons? Hydrogen bonding > dipole-dipole > London dispersion forces The more intermolecular forces, the stronger the interaction and the higher the boiling points EXAMPLE PROBLEMS Identify the intermolecular forces in the following substances: He, CH3(CH2)4CH3, NF3, (CH3)2O, CH3F, CH3CH2OH Draw Lewis structures as needed He – London forces only CH3(CH2)4CH3 symmetrical = non-polar so London forces only EXAMPLE PROBLEMS Identify the intermolecular forces in the following substances: He, CH3(CH2)4CH3, NF3, (CH3)2O, CH3F, CH3CH2OH NF3 Polar so London and Dipoledipole forces (CH3)2O Polar so London and Dipole-dipole forces EXAMPLE PROBLEMS Identify the intermolecular forces in the following substances: He, CH3(CH2)4CH3, NF3, (CH3)2O, CH3F, CH3CH2OH CH3F Polar so London and Dipoledipole forces CH3CH2OH Polar w/ Hbond so London, Dipoledipole forces, and Hbond EXAMPLE PROBLEMS Place sulfur (S8), chlorine, and argon in order of increasing boiling points. Explain your order. Argon<Chlorine<Sulfur All non-polar so only force is London force Sulfur (S8) has highest B.P. due to it being the largest molecule (molecular mass) and ∴ the strongest London force, followed by Cl2 and then Ar EXAMPLE PROBLEMS Compare the boiling points of propane (CH3CH2CH3), methoxymethane (CH3OCH3) and ethanol (CH3CH2OH) CH3CH2CH3< CH3OCH3 < CH3CH2OH CH3CH2CH3 non-polar London only CH3OCH3 polar – London and dipole-dipole CH3CH2OH London, dipole-dipole and H-bond EXAMPLE PROBLEMS Melting point is a measure of the difference in strength of the forces between particles between the solid and liquid state. Order the following from lowest to highest melting point: HI, HCl, HBr, HF. HF<HCl<HBr<HI M.P for HF is -118 oC, for HCl is -114.2 oC, for HBr is -86.8 oC, for HI is -50.80 oC The larger the atomic size, the more energy it requires to melt the molecule. The larger the size, the more electrons there are moving randomly around, so the more likely to polarize and probably stronger London forces occurring between each molecule. Let’s See If You Have It 1. Which attractions are stronger: intermolecular or 2. 3. 4. 5. intramolecular? Suggest some ways that London forces are different from dipole-dipole forces. Which would have a lower boiling point: O2 or F2? Explain. Which would have a lower boiling point: NO or O2? Explain. Which would you expect to have the higher melting point (or boiling point): C8H18 or C4H10? Explain. Let’s See If You Have It 6. What two factors causes hydrogen bonds to be so much stronger than typical dipole-dipole bonds? 7. What kind(s) of intermolecular forces are present in the following substances: NH3, SF6, PCl3, LiCl, HBr, CO2 (hint: consider EN and molecular shape/polarity) Let’s See If You Have It 1. Intramolecular are stronger. 2. London forces Are present in all compounds Can occur between atoms or molecules Are due to electron movement not to EN Are transient in nature (dipole-dipole are more permanent). London forces are weaker F2 would be lower because it is smaller. Larger atoms/molecules can have their electron clouds more easily deformed and thus have stronger London attractions and higher melting/boiling points. O2 because it has only London forces. NO has a small EN, giving it small dipoles. 3. 4. Let’s See If You Have It 5. 6. 7. C8H18 would have the higher melting/boiling point. This is a result of the many more sites available for London forces to form (larger molecule) a large EN and the small sizes of atoms NH3: Hydrogen bonding (H + N), dipole-dipole London. SF6: London only (it is symmetrical & non-polar). PCl3: EN=2.9-2.1= 0.8. Dipole-dipole, London. LiCl: EN=2.9-1.0=1.9 Ionic, (London). HBr: EN=2.8-2.1=0.7. Dipole-dipole, London. CO2: London only (it is symmetrical & non-polar) Let’s See If You Have It 5. 6. 7. C8H18 would have the higher melting/boiling point. This is a result of the many more sites available for London forces to form (larger molecule) a large EN and the small sizes of atoms NH3: Hydrogen bonding (H + N), dipole-dipole London. SF6: London only (it is symmetrical & non-polar). PCl3: EN=2.9-2.1= 0.8. Dipole-dipole, London. LiCl: EN=2.9-1.0=1.9 Ionic, (London). HBr: EN=2.8-2.1=0.7. Dipole-dipole, London. CO2: London only (it is symmetrical & non-polar) Reading Pages 122 - 132 Look over worked examples