practice exam 2 - Chemistry at Winthrop University
advertisement

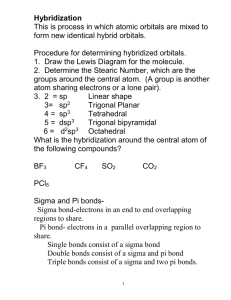
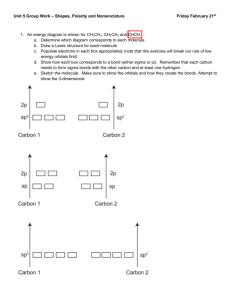
Dr. Harris Chemistry 105-002 Exam 2 10/17/13 Name Directions: Show all work. Show all units. 1. Determine the number of pi and sigma bonds in the following molecule: NH2CH2CO2CN (2) a.) 9σ, 2π b.) 10σ, 3π c.) 10σ, 2π d.) 9σ, 3π 2. Which of the following are weak electrolytes? (Select all that apply) (2) a.) FeCl3 b.) MgCO3 d.) NH3 e.) CsClO4 c.) (NH4)2SO4 3. A five coordinate molecule containing 2 bonding domains and 3 lone pair would assume which of the following geometries? (2) a.) seesaw b.) t-shape d.) bent e.) linear c.) trigonal bipyramid 4. When four atomic orbitals are hybridized, four hybrid orbitals are formed. (2) a.) true b.) false c.) can not determine 5. An atom with four hybrid orbitals must make four sigma bonds. (2) a.) true b.) false c.) can not determine 6. 100 mL of HBr is mixed with 100 mL of LiOH. A base indicator is added to the resulting solution, causing it to turn pink. Which of the following statements are correct: (2) a.) [HBr] = [LiOH] b.) [HBr] > [LiOH] c.) [HBr] < [LiOH] d.) can not determine 7. In the reaction: H2 (g) + Cl2 (g) H2O (L) 2HCl (aq), hydrogen is: (3) a.) oxidized, reducing agent b.) oxidized, oxidizing agent c.) reduced, oxidizing agent d.) reduced, reducing agent 8. The number of hydrogen atoms in 1 g of ammonium hydroxide. (3) a.) 1.72 x 1021 b.) 3.44 x 1021 c.) 5.16 x 1021 d.) 6.88 x 1021 e.) 8.60 x 1021 f.) none of the above /18 9. In full detail, explain the differences between ionic and covalent bonding. (4) 10. Why is it necessary for an atom to hybridize its lone pair (e.g. H2O) ? (4) 11. A methane molecule (CH4) has four electron domains, and its geometry is tetrahedral. The bond angles within the methane molecule are 109.5o. An ammonia molecule (NH3) also has four electron domains, but its geometry is trigonal pyramidal, with bond angles of 107.5o. Why are these structures different? (4) 12. Review Lewis Structure Quiz. 13. A sample of a substance containing only aluminum and sulfur is found to be 36% aluminum by mass. Given that the molecular mass of the substance is 450 g/mol, determine the molecular formula. (7) 14. 120 mL of 0.20M aqueous cobalt (III) acetate is added to 125 mL 0.24 M potassium sulfide. The system immediately undergoes a double replacement reaction. A solid, cobalt (III) sulfide, is formed. The remaining species are aqueous spectators. a. Write a balanced reaction, including phases. Given that 0.73g of cobalt (III) sulfate form, determine the percent yield of the reaction. (15) b. Determine the molarity of the aqueous product. (7) 15a. As part of a community service project, you spend a day at a local middle school, assisting 7th graders in chemistry lab. A student needs to prepare 100 mL of an aqueous 0.50M stock solution of lithium chlorate. How would you have the student prepare this solution? Keep in mind that you are explaining this to a 7th grader. (6) 15b. Using the stock solution above, the student must then create an additional 100 mL of a 0.10M solution. How would you have the student prepare this solution? (6)BONUS 16. Consider the molecule nitrate. Draw the orbital diagram of the atoms, making sure to clearly indicate any energetic differences between the valence atomic orbitals. Illustrate the hybridization of the atoms by showing the orbitals involved, and their merger to form hybrid orbitals. Sketch the overlap of these orbitals. Label the sigma and pi bonds. (7.5) 17. Arrange the following in order of increasing boiling temperature: SO3, I2, SO2, LiF, HCN, ClF3, H2O, CH3NH2, CH3SH Equations and constants M= 𝑚𝑜𝑙𝑒𝑠 𝑠𝑜𝑙𝑢𝑡𝑒 𝐿𝑖𝑡𝑒𝑟𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 moles = MV M1V1 = M2V2 NA = 6.02 x 1023 mol-1 %yield = 𝑎𝑐𝑡𝑢𝑎𝑙 𝑡ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙 𝑥 100%