Chapter 1 Structure and Bonding
advertisement

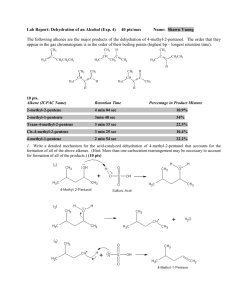
Chapter 11 Alkenes and IR I. H H H Alkene Nomenclature A. Unsaturation 1) Alkanes: CnH2n+2 H C C C H H H H H 2) Alkenes: CnH2n H C C H 3) C H H H Degree of Unsaturation a) Tells us how many rings and double bonds in molecule b) Hsat = 2C + 2 – X + N (Ignore O, S) c) Degree of Unsaturation = (Hsat – Hact)/2 d) Example: C5H8NOCl i. Hsat = 2(5) + 2 – 1 + 1 = 12 ii. (Hsat – Hact)/2 = (12 – 8)/2 = 2 degrees of unsaturation O H H Cl H C N C C C H H H C H H B. Nomenclature 1. Common Names end with –ylene H a. Ethylene H H C b. 2. 3. 4. Propylene H H C C C H H C H H H IUPAC: Replace –ane of an alkane with –ene of an alkene a. Ethene b. Propene Alkenes follow alkane nomenclature, with double bond location numbered closest to end H H a. 1-butene CH2 CH3 C CH3 C C b. 2-butene H3C C H c. Cylclohexene H H Substituents named as prefixes with lowest numbers a. 3-methyl-1-pentene b. 3-methylcyclohexene 5. Disubstituted Alkenes can be cis or trans streoisomers CH3 CH3 H CH3 a. cis-2-butene C C C C H H b. trans-2-butene H CH3 c. Cycloalkenes cis unless large 6. Tri- or Tetra-substituted Alkenes can be E or Z stereoisomers a. Use priorities from R/S nomenclature b. Assign 1-2 on each carbon c. Move from 1-2-1-2 to trace out an E or Z CH3CH2 CH2CH2CH3 Br F C C C C ClCH2CH2 CH3 F H E-1-chloro-3-ethyl-4-methyl-3-heptene Z-1-bromo-1,2-difluoroethene 7. Alcohols have priority over alkene in numbering: Alkenol H3C OH Cl 2-propen-1-ol CH CHCH2CH3 Z-5-chloro-3-ethyl-4-hexen-2-ol C C CH3 8. OH H H Alkene substituents are named alkenyl ethenylcyclohexane CH2 R 2-propenyl- R trans-1-propenyl- C C CH3 H II. Pi-bonding in Alkenes A. The p-bond 1) sp2 hybridization results in 120o angles 2) H1s-Csp2 overlap gives the CH s-bonds 3) Csp2—Csp2 overlap gives the C—C s-bond 4) Cp—Cp overlap gives the C—C p-bond B. Bond Strength 1) Bond strength is proportional to orbital overlap 2) The s-bond in ethene is very strong because of good overlap 3) The p-bond in ethene is fairly weak because of poor overlap 4) Overall, the double bond is stronger than a C—C single bond 5) The weak p-bond will be the reactive part of the molecule 6) Orbital and Energy level diagrams for ethene 7) Thermal Isomerization tells us the p-bond energy a. cis/trans interconversion must go through broken p-bond T.S. b. c. d. 8) Ea = 65 kcal/mol should be about the same as the p-bond strength The s-bond is slightly stronger than alkane due to better sp2 overlap C—H bonds are also stronger than in alkanes (110 kcal/mol) Radical H-atom abstraction doesn’t occur in alkenes because of the strong C-H bonds. The chemistry is dominated by the weak p-bond. III. Physical properties of Alkenes A. B. Boiling points are about like alkanes Melting points depend on the isomer 1) cis-alkenes have a U-shape that disrupts packing in the solid, giving lower temperatures (Vegetable oils have cis-alkenes) 2) trans-alkenes have melting points close to the alkanes C. Polarization 1) Alkenes are more polar than alkanes due to more e-withdrawing sp2 hybrid orbitals (more s-character draws e- closer to nucleus) 2) cis-alkenes are more polar than trans-alkenes due to their shape H H H R C C C C CH3 D. H CH3 R Acidity of alkenes > alkanes, again because of the greater s-character of sp2 hybrid orbitals. 1) Ethane pKa = 50 2) Ethene pKa = 44 IV. NMR of Alkene A. p-electrons deshield hydrogens 1) Alkane H 1.0 ppm 2) Alkene H 5-6 ppm 3) Spectrum of an alkene B. Coupling in Alkenes depends on the isomer C. 13C NMR of alkenes gives peaks at 100-150 ppm due to deshielding H3C 122.8 CH3 C C H3C 18.9 CH3 H 123.7 C H3C 12.3 H C132.7 CH2CH3 20.5 14.0