Fig 20.UN, p.504

Lipids
• Lipids are a heterogeneous class of naturally occurring organic compounds classified together on the basis of common solubility properties
– they are insoluble in water, but soluble in aprotic organic solvents, including diethyl ether, methylene chloride, and acetone
• Lipids include
– triglycerides, phospholipids, prostaglandins, prostacyclins, and fat-soluble vitamins
– cholesterol, steroid hormones, and bile acids
..\Word\TYPES OF LIPIDS.doc
Fig 20.1, p.501
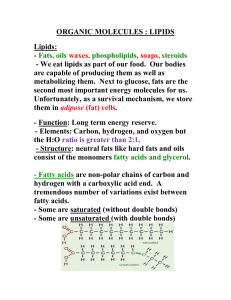
Fatty Acids
• Fatty acid
: a long, unbranched chain carboxylic acid, most commonly of 12 to 20 carbons, derived from hydrolysis of animal fats, vegetable oils, or the phospholipids of biological membranes
Table 20.1, p.496
Fatty Acids
• Among the fatty acids most abundant in plants and animals
– nearly all have an even number of carbon atoms, most between 12 and 20, in an unbranched chain
– the three most abundant are palmitic, stearic acid, and oleic acid
– in most unsaturated fatty acids, the cis isomer predominates; the trans isomer is rare
– unsaturated fatty acids have lower melting points than their saturated counterparts; the greater the degree of unsaturation, the lower the melting point
Fig. 21.UN, p.496
Fig. 21.UN, p.496
Triglycerides
• Triglyceride
: an ester of glycerol with three fatty acids
O
O CH
2
OCR
R'CO CH O
CH
2
OCR''
A triacylglycerol
(a triglyceride)
1 . NaOH, H
2 . HCl, H
2
O
2
O
CH
HO CH
2
OH
CH
2
OH
1,2,3-Propanetriol
(Glycerol, glycerin)
+
RCO
2
H
R'CO
2
H
R''CO
2
H
Fatty acids
Fig. 21.UN, p.497
Triglycerides
• Physical properties depend on the fatty acid components
– melting point increases as the number of carbons in its hydrocarbon chains increases and as the number of double bonds decreases
– triglycerides rich in unsaturated fatty acids are generally liquid at room temperature and are called oils
– triglycerides rich in saturated fatty acids are generally semisolids or solids at room temperature and are called fats
Triglycerides
• The lower melting points of triglycerides rich in unsaturated fatty acids are related to differences in their three-dimensional shape
– hydrocarbon chains of saturated fatty acids can lie parallel, there are strong dispersion forces between their chains; these triglycerides pack into well-ordered, compact crystalline forms and have melting points above room temperature
– because of the cis configuration of the double bonds in unsaturated fatty acids, their hydrocarbon chains have a less ordered structure and dispersion forces between hydrocarbon chains are weaker; these triglycerides have melting points below room temperature
Table 20.2, p.498
Soaps and Detergents
• Natural soaps are prepared by boiling lard or other animal fat with NaOH, in a reaction called saponification (Latin, sapo , soap)
O
O
CH
2
OCR
RCO CH +
O
CH
2
OCR
A triglyceride
(a triester of glycerol)
3 NaOH saponification
CH
2
OH
CHOH +
CH
2
OH
1,2,3-Propanetriol
(Glycerol; Glycerin)
O
3 RCO
-
Na
+
Sodium soaps
Soaps and Detergents
• Soaps clean by acting as emulsifying agents
– the long hydrophobic hydrocarbon chains of soaps are insoluble in water and tend to cluster in such a way as to minimize their contact with water
– the polar hydrophilic carboxylate groups, on the other hand, tend to remain in contact with the surrounding water molecules
– driven by these two forces, soap molecules spontaneously cluster into micelles
Chem Connect 20C, p.500
Soaps and Detergents
• Micelle
: a spherical arrangement of organic molecules in water clustered so that their hydrophobic parts are buried inside the sphere and their hydrophilic parts are on the surface of the sphere and in contact with water
• When soap is mixed with water-insoluble grease, oil, and fat stains, the nonpolar parts of the soap micelles “dissolve” these nonpolar dirt molecules and they are carried away in the polar wash water
Soaps and Detergents
• Soaps form water-insoluble salts when used in water containing Ca(II), Mg(II), and
Fe(III) ions ( hard water )
2 CH
3
(CH
2
)
14
CO
2
-
Na
+
A s odium soap
(soluble in water as micelles )
+ Ca
2+
[CH
3
(CH
2
)
14
CO
2
-
]
2
Ca
2+
Calcium salt of a fatty acid
(insoluble in water)
+ 2 Na
+
Synthetic Detergents
• The design criteria for a good detergent are
– a long hydrocarbon tail of 12 to 20 carbons
– a polar head group that does not form insoluble salts with Ca(II), Mg(II), or Fe(III) ions
• The most widely used synthetic detergents are the linear alkylbenzene sulfonates
(LAS)
Chem Connect 20C, p.500
Phospholipids
• Phospholipids are the second most abundant group of naturally occurring lipids
– they are found almost exclusively in plant and animal membranes, which typically consist of 40% -50% phospholipids and 50% - 60% proteins
– the most abundant phospholipids are derived from phosphatidic acid, a molecule in which glycerol is esterified with two molecules of fatty acid and one of phosphoric acid
– the three most abundant fatty acids in phosphatidic acids are palmitic acid (16:0), stearic acid (18:0), and oleic acid (18:1)
Phospholipids
palmitic acid
O
O
O
CH
2
-O-P-O
CH
O
-
-
O
O CH
2 glycerol
• further esterification with a low-molecular weight alcohol gives a phospholipid
– among the most common of these low-molecularweight alcohols are
Phospholipids
Name and Formula Name of Phospholipid ethanolamine
HOCH
2
CH
2
NH
2 phosphatidylethanolamine
(cephalin) choline
HOCH
2
+
CH
2
N(CH
3
)
3 serine
HOCH
2
CHCO
2
+
NH
3 inositol
-
HO
HO
HO
OH
OH
OH phosphatidylcholine
(lecithin) phosphatidylserine phosphatidylinositol
Fig 20.UN, p.504
Phospholipids
• A lecithin stearic acid choline
O
O
O P OCH
O
-
CH
2
2
CH
2
+
N(CH
3
)
3
O CH
O
O CH
2 glycerol palmitic acid
• In aqueous solution, phospholipids spontaneously form into a lipid bilayer, with a back-to-back arrangement of lipid monolayers
Fig 20.3, p.504
Biological Membranes
• Fluid mosaic model
: a biological membrane consists of a phospholipid bilayer with proteins, carbohydrates, and other lipids embedded on the surface and in the bilayer
– fluid signifies that the protein components of membranes “float”in the bilayer and can move freely along the plane of the membrane
– mosaic signifies that the various components of the membrane exist side-by-side, as discrete units rather than combining to form new molecules and ions
Fig 20.2, p.503
Chem Connect 20D, p.502
Go to gob – Ch 20 – complex lipids tutorial
D:\GOB.exe
Fig 20.UN, p.505
Fig 20.UN, p.505
Chem Connect 20E, p.506
Steroids
• Steroids
: a group of plant and animal lipids that have this tetracyclic ring structure
C D
A B
• The features common to the ring system of most naturally occurring steroids are illustrated on the next screen
Steroids
CH
3
H
CH
3
H
H
H
Cholesterol
HO
H
3
C
H
3
C
H
H H
Fig 20.6, p.513
Androgens
• Androgens - male sex hormones
– synthesized in the testes
– responsible for the development of male secondary sex characteristics
H
3
C
OH
H
H
3
C
H
3
C H H
3
C H
O
H H H H
O HO
Tes tos terone Androsterone
Anabolic Steroids
O
• Among the synthetic anabolic steroids are
2
H
3
C
1
H
H
3
C
OH
CH
3
17
H
H
H
3
C
OH
CH
3
H
Methandros tenolone
H
3
C
HN
N
A H H
Stanozolol
H
Estrogens
• Estrogens - female sex hormones
– synthesized in the ovaries
– responsible for the development of female secondary sex characteristics and control of the menstrual cycle
CH
3
H
3
C
C=O
H
H
3
C O
H
3
C H H
H H H H
O HO
Progesterone Es trone
Synthetic Estrogens
• Progesterone-like analogs are used in oral contraceptives
H
3
C
HO C CH
"Nor" refers to the abs ence of a methyl group here.
The methyl group is pres ent in ethindrone
H
H
H
H
O
Norethindrone
O
Glucorticoid Hormones
– synthesized in the adrenal cortex
– regulate metabolism of carbohydrates
– decrease inflammation
– involved in the reaction to stress
H
3
C
CH
2
OH
C=O
OH HO
H
3
C
O
H
3
C H H
3
C H
CH
2
OH
C=O
OH
H H H H
O
Cortisone Cortisol
Prostaglandins
• Prostaglandins
: a family of compounds that have the 20-carbon skeleton of prostanoic acid
9
8
7
6
5
4
3
2
1
CO
2
H
10
11
12
13
14
15
16
17
Pros tanoic acid
18
19
20
Chem Connect 20I, p.519
Fig 20.UN, p.520
Prostaglandins
• Prostaglandins are not stored in tissues as such, but are synthesized from membranebound 20-carbon polyunsaturated fatty acids in response to specific physiological triggers
– one such polyunsaturated fatty acid is arachidonic acid
9 8
6 5
CO
2
H
11 12 14 15
Arachidonic acid
Prostaglandins
• Among the prostaglandins synthesized biochemically from arachidonic acid are
O
9
CO
2
H
PGE
2
15
HO
11
HO H
HO
9
PGF
2
CO
2
H
15
HO
11
HO H
Eicosanoids
• The prostaglandins are members of an even larger family of compounds called eicosanoids , all of which contain 20 carbons and are derived from polyunsaturated fatty acids
H
H
11
9
8
O
O
15
12 14
13
OH
Thromboxane A
2
(a potent vas oconstrictor)
1
CO
2
H
20