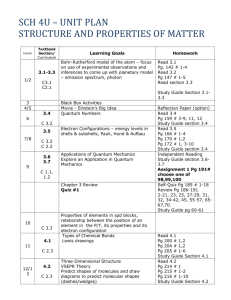
Note

Molecular Structure
Molecular
Geometry
III
A. VSEPR Theory
V alence Shell Electron Pair
Repulsion Theory
Electron pairs orient themselves so that valence electrons are as far apart as possible
A. VSEPR Theory
Types of e Pairs
Bonding pairs - form bonds
Lone pairs - nonbonding e -
Lone pairs repel more strongly than bonding pairs!!!
A. VSEPR Theory
Lone pairs reduce the bond angle between atoms.
Bond Angle
B. Determining Molecular Shape
Draw the Lewis Diagram.
Count up e pairs on central atom.
double/triple bonds = ONE pair
Shape is determined by the # of bonding pairs and lone pairs.
Know the 8 common shapes
& their bond angles!
C. Common Molecular Shapes
2 total electron pairs
*2 bonding pairs
*0 lone pairs
BeH
2
Note: Beryllium does not follow the octet rule. Beryllium is complete with 4 valence electrons
LINEAR
180 °
C. Common Molecular Shapes
3 total electron pairs
*3 bonding pairs
*0 lone pairs
BF
3 TRIGONAL PLANAR
Note: Boron is also an exception to the octet rule. Boron is complete with
6 valence electrons
120 °
C. Common Molecular Shapes
3 total pairs
*2 bonding pairs
*1 lone pair
SO
2 BENT
<120 °
C. Common Molecular Shapes
4 total pairs
*4 bonding pairs
*0 lone pairs
CH
4
TETRAHEDRAL
109.5
°
C. Common Molecular Shapes
4 total pairs
*3 bonding pairs
*1 lone pair
NH
3
TRIGONAL PYRAMIDAL
107 °
C. Common Molecular Shapes
4 total pairs
*2 bonding pairs
*2 lone pairs
H
2
O
BENT
104.5
°
C. Common Molecular Shapes
5 total pairs
*5 bonding pairs
*0 lone
PCl
5
TRIGONAL
BIPYRAMIDAL
120 °/90°
C. Common Molecular Shapes
6 total pairs
*6 bonding pairs
*0 lone pairs
SF
6
OCTAHEDRAL
90 °
D. Examples n
PF
3
4 total
3 bond
1 lone
F P F
F
TRIGONAL
PYRAMIDAL
107 °
D. Examples n
CO
2
2 total
2 bonding pairs
0 lone
O C O
LINEAR
180 °
Molecular Structure
Molecular
Polarity
III
Molecular Polarity
• Polar Molecule : Molecule that has a partially positive end and a partially negative end. (It has electrical poles)
• There is an uneven distribution of electrons in the molecule
A. Dipole Moment
Direction of the polar bond in a molecule.
Arrow points toward the more electronegative atom.
+ H Cl
-
B. Determining Molecular Polarity
Depends on:
dipole moments
molecular shape
B. Determining Molecular Polarity
Nonpolar Molecules
Dipole moments are symmetrical and cancel out.
F
BF
3
B
F
F
B. Determining Molecular Polarity
Polar Molecules
Dipole moments are asymmetrical and don’t cancel .
.. ..
O
H
2
O
H H net dipole moment
B. Determining Molecular Polarity
Polar molecules have...
asymmetrical shape (lone pairs) or
asymmetrical atoms (attached atoms are different)
B. Determining Molecular
Polarity
Nonpolar Molecules have…
No lone pairs
All attached atoms are the same