SCH4U-Unit One-Structure & Properties
advertisement
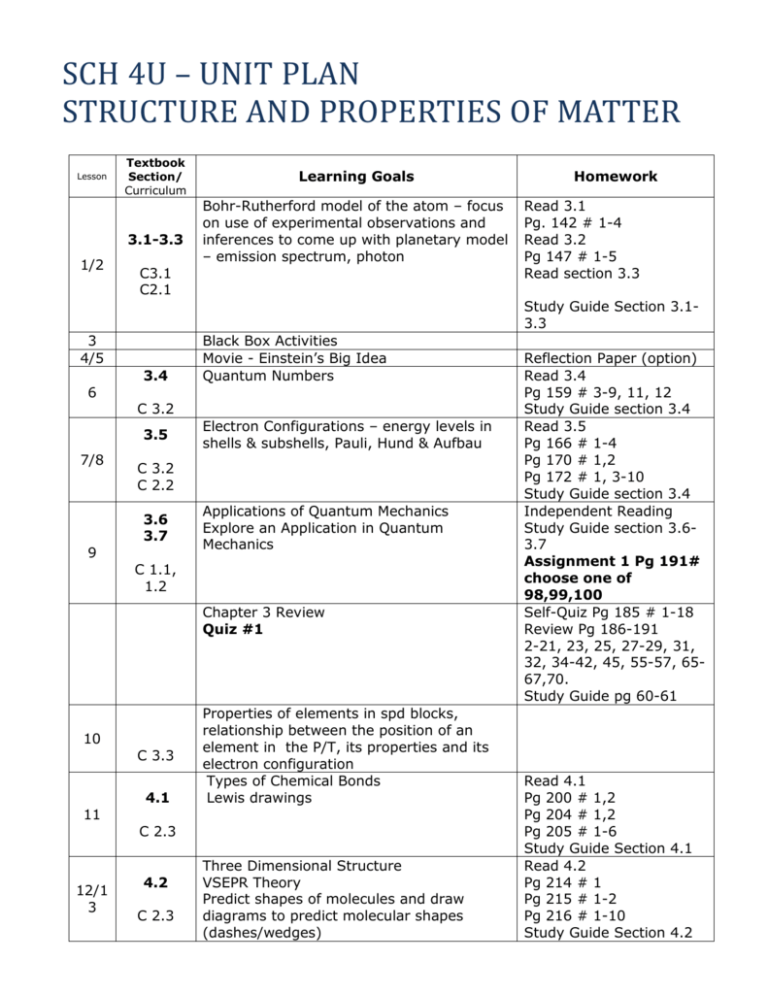
SCH 4U – UNIT PLAN STRUCTURE AND PROPERTIES OF MATTER Lesson Textbook Section/ Curriculum 3.1-3.3 1/2 3.4 9 Black Box Activities Movie - Einstein’s Big Idea Quantum Numbers Electron Configurations – energy levels in shells & subshells, Pauli, Hund & Aufbau C 3.2 C 2.2 3.6 3.7 Applications of Quantum Mechanics Explore an Application in Quantum Mechanics C 1.1, 1.2 Chapter 3 Review Quiz #1 10 C 3.3 11 12/1 3 4.1 Properties of elements in spd blocks, relationship between the position of an element in the P/T, its properties and its electron configuration Types of Chemical Bonds Lewis drawings C 2.3 4.2 C 2.3 Homework Read 3.1 Pg. 142 # 1-4 Read 3.2 Pg 147 # 1-5 Read section 3.3 Study Guide Section 3.13.3 C 3.2 3.5 7/8 Bohr-Rutherford model of the atom – focus on use of experimental observations and inferences to come up with planetary model – emission spectrum, photon C3.1 C2.1 3 4/5 6 Learning Goals Three Dimensional Structure VSEPR Theory Predict shapes of molecules and draw diagrams to predict molecular shapes (dashes/wedges) Reflection Paper (option) Read 3.4 Pg 159 # 3-9, 11, 12 Study Guide section 3.4 Read 3.5 Pg 166 # 1-4 Pg 170 # 1,2 Pg 172 # 1, 3-10 Study Guide section 3.4 Independent Reading Study Guide section 3.63.7 Assignment 1 Pg 191# choose one of 98,99,100 Self-Quiz Pg 185 # 1-18 Review Pg 186-191 2-21, 23, 25, 27-29, 31, 32, 34-42, 45, 55-57, 6567,70. Study Guide pg 60-61 Read 4.1 Pg 200 # 1,2 Pg 204 # 1,2 Pg 205 # 1-6 Study Guide Section 4.1 Read 4.2 Pg 214 # 1 Pg 215 # 1-2 Pg 216 # 1-10 Study Guide Section 4.2 4.3 14 C2.4 4.4 15 4.5 C 2.4 16/1 7 4.6 4.7 18 19 C 3.4 C 2.6 A1.1, A1.2, A1.4 4.8 20 Molecule Building Lab Investigation 4.2.1 Electronegativity and Bond Polarity (dipole) C 2.5 Describe a Canadian contribution to the field of atomic and molecular theory Molecular Polarity Predict polarity of various chemical compounds based on their molecular shapes and EN (bond polarity vs. molecular polarity) Quantum Mechanics and Bonding: Hybridization Intermolecular Forces The role they play in explaining the physical properties of a solid or liquid (solubility, boiling point, melting point, melting point suppression, hardness, conductivity, surface tension)depending on the particles present and the types of inter/intramolecular forces – covalent, ionic bonding, Van der Waals forces, Hydrogen bonding, metallic bonding Prime Suspect Lab LAB – Observe and analyze physical properties of various substances and determine the type of bonding present in each substance LAB TEST The Structure and Properties of Solids – predict type of solid (ionic, molecular, covalent network, metallic) formed by a given substance UNIT TEST 21 Unit Culminating Assignment Pg 255 Read section 4.3 Pg 220 # 1,2 Pg 221 # 1-10 Study Guide Section 4.3 Read section 4.4 Pg 223 # 1-5 Study Guide Section 4.4 Read section 4.5 Pg 227-228 # 1-3 Pg 229 # 1-7 Study Guide Section 4.5 Read section 4.6 Pg 238 # 1-10 Study Guide Section 4.6 Read section 4.7 Pg 247 # 1-8 Study Guide Section 4.7 Read section 4.8 Pg 254 # 1-4, 6, 7 Study Guide section 4.8 Chapter 4 Review Pg 261 – Self-Quiz Pg 262 # 1-10, 13-23, 26-30, 33, 35-38, 43-60, 62-64, 68, 71-73, Study Guide pg 84-86 Unit 2 Review Pg 270 – Unit 2 Self-Quiz Pg 272 – 279 -Choose questions that you think you need practice with. Study Guide pg 87-88 TBD