Ion Sheet with Alkane and acid naming
advertisement

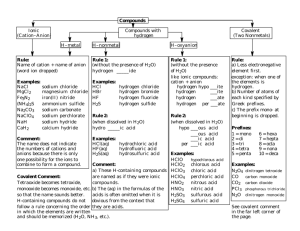
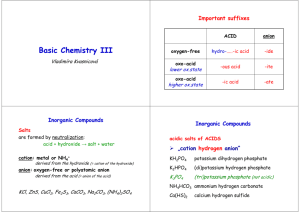
TABLE OF COMMON IONS POSITIVE IONS or CATIONS +1 ammonium (NH4+) cesium (Cs+) copper (I) (Cu+) gold(I) (Au+) hydrogen (H+) lithium (Li+) potassium (K+) rubidium (Rb+) silver (Ag+) sodium (Na+) - Name (formula or symbolcharge) +2 barium (Ba2+) beryllium (Be2+) cadmium (Cd2+) calcium (Ca2+) chromium (II) (Cr2+) cobalt (II) (Co2+) copper (II) (Cu2+) iron (II) (Fe2+) lead (II) (Pb2+) magnesium (Mg2+) manganese (II) (Mn2+) mercury (I) (Hg22+) mercury (II) (Hg2+) nickel (II) (Ni2+) platinum (II) (Pt2+) strontium (Sr2+) tin (II) (Sn2+) zinc (Zn2+) +3 aluminum (Al3+) chromium (III) (Cr3+) cobalt (III) (Co3+) gallium (Ga3+) gold (III) (Au3+) iron (III) (Fe3+) nickel (III) (Ni3+) +4 lead (IV) (Pb4+) manganese (IV) (Mn4+) platinum (IV) (Pt4+) tin (IV) (Sn4+) NEGATIVE IONS or ANIONS - Name (formula or symbolcharge) -1 -2 acetate (C2H3O2 ) carbonate (CO32-) bromate (BrO3 ) chromate (CrO42-) bromide (Br-) dichromate (Cr2O72-) chlorate (ClO3-) hydrogen phosphate (HPO42-) chloride (Cl ) oxalate (C2O42-) chlorite (ClO2-) oxide (O2-) cyanate (CNO ) peroxide (O22-) cyanide (CN ) selenide (Se2-) dihydrogen phosphate (H2PO4-) silicate (SiO32-) fluoride (F ) sulfate (SO42-) hydrogen carbonate (HCO3-) sulfide (S2-) hydrogen sulfate (HSO4-) sulfite (SO32-) hydrogen sulfite (HSO3 ) telluride (Te2-) hydroxide (OH-) thiosulfate (S2O32-) hypochlorite (ClO ) hypobromite (BrO-) -3 iodate (IO3 ) iodide (I-) arsenate (AsO43-) nitrate (NO3-) borate (BO33-) nitrite (NO2-) nitride (N3-) perchlorate (ClO4 ) phosphate (PO43-) periodate (IO4-) phosphide (P3-) permanganate (MnO4 ) thiocynate (SCN-) Ending of anion attached to hydrogen Name of acid ________ ide hydro ________ ic acid ________ ate ___________ ic acid ________ ite ___________ ous acid All acids contain hydrogen. The hydrogen is written at the beginning of the formula. Examples: Question: What is the formula of nitric acid? Answer: All acids contain hydrogen. Write H+ as the symbol for hydrogen. Nitric acid means that the anion attached to the hydrogen is the nitrate. Follow the H+ with the formula of the nitrate ion, NO3-. Complete the subscript for H+, if necessary, to write the correct formula. HNO3 is the answer. Question: What is the name of HBr? Answer: Since the compound begins with an H, we will assume it is an acid. The H is followed by Br- which is called the bromide ion. Since bromide ends in ide, the acid is called hydrobromic acid. Number of atoms 1 2 3 4 5 6 7 8 9 10 prefix mono di tri tetra penta hexa hepta octa nona deca Number of carbon atoms 1 2 3 4 5 6 7 8 9 10 root of name meth eth prop but pent hex hept oct non dec