Histology Ch 14 466-474
advertisement

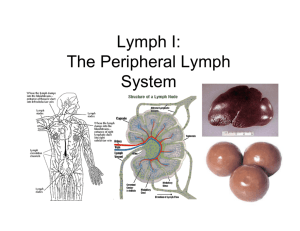
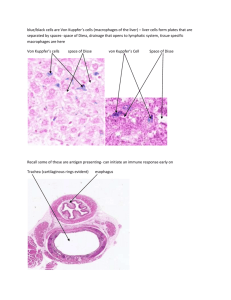
Histology Ch 14 466-474 Thymus -Thymus is a bilobed organ in superior mediastinum anterior to heart; develops from 3rd and 4th branchial pouch. Developmentally, epithelium invaginates and thymus grows caudally as a tubular projection of endodermal epithelium into mediastinum; tip proliferates and becomes disconnected from branchial epithelium -Multipotential lymphoid stem cells (CFU-Ls) – from bone marrow become immunocompetent T cells that invade epithelial rudiment of thymus and occupy spaces between epithelial cells, so thymus develops as a lymphoepithelial organ -thymus fully formed and functional at birth and persists until puberty, when T cell differentiation and proliferation are reduced and thymus replaced with adipose tissue, but can be restimulated under conditions that demand rapid T cell proliferation General Architecture of Thymus – connective tissue surrounds thymus and divides it into lobules; connective tissue capsule from which trabeculae extend into parenchyma of organ containing blood vessels, EFFERENT lymphatic vessels, and nerves -connective tissue of thymus has collagen fibers, fibroblasts, granulocytes, lymphocytes, mast cells, adipose cells, and macrophages -trabeculae establish thymic lobules, which are not true lobules, but rather cortical caps over portions of highly convoluted continuous inner medullary tissue -Thymic parenchyma contains developing T cells in an extensive meshwork formed by epithelioreticular cells -the thymic cortex is basophilic and contains developing T cells (thymocytes) with intensely stained nuclei, and occupy spaces within meshwork of epithelioreticular cells, whichprovide framework for developing T cells and correspond to reticular cells and their fibers, but also have intercellular junctions and intermediate filaments (epithelial nature). -macrophages are also inside cortical cells -T cells arise from CFU-L cells in bone marrow -Six types of epithelioreticular cells: 3 in cortex (I-III) and 3 in medulla (IV – VI) 1. Type I Epithelioreticular cells - boundary of cortex and CT capsule and between cortical parenchyma and trabeculae, and surround blood vessels; function to separate parenchyma from CT 2. Type II Epithelioreticular cells – within cortex, contain, macula adherens (desmosomes) that join long, cytoplasmic processes of adjacent cells; contain intermediate filaments; function to COMPARTMENTALIZE CORTEX INTO AREAS for T CELL DEVELOPMENT. Type II cells contain MHCI and MHCII cells for thymic cell education 3. Type III Epithelioreticular cells – boundary of cortex and medulla and create functional barrier between cortex and medulla and possess MHCI and II molecules 4. Macrophages – reside in cortex, responsible for phagocytosis of T cells that fail requirements; 98% of T cells undergo apoptosis and are phagocytized, called PAS cells -crosstalk – phenomenon where T cells at different stages of differentiation control microarchitecture of thymic epithelioreticular cells -thymic or Hassall’s corpuscles are DISTINGUISHING FEATURE OF MEDULLA** -Thymic medulla – inner portion of parenchyma, contains epithelioreticular cells and loosely packed T cells; stains less intensely than cortex because it contains LARGE lymphocytes which have pale-staining nuclei -thymic medulla contains 3 types of epithelioreticular cells: 1. Type IV Epithelioreticular cells – between cortex/medulla close to type III, and possess sheetlike processes with occluding junctions between cells to help create barrier at corticomedullary junction 2. Type V Epithelioreticular cells – located all over medulla, like type II cells are connected by desmosomes to provide medullary framework and compartmentalize T cells 3. Type VI Epithelioreticular cells – form MOST CHARACTERISTIC FEATURE OF MEDULLA, the thymic (Hassall’s) corpuscles, which are isolated masses of closely packed, concentrically arranged TYPE VI epithelioreticular cells with flattened nuclei a. These corpuscles reveal keratohyalin granules, intermediate filaments, and lipid droplets; cells joined by desmosomes b. Center of corpuscle may show keratinization c. Corpuscles are unique, may be antigenically distinct, and functionally different (produce interleukins) -blood vessels pass from trabeculae to enter parenchyma of thymus; vessels enter medulla from deeper parts of trabeculae and carry sheath of CT with them Blood-Thymus Barrier and T-Cell education – blood thymus barrier protects developing lymphocytes in thymus from antigen exposure -T cells reaching cortex are prevented from antigen contact by blood-thymus barrier, composed of, from the LUMEN of BLOOD VESSELS Outward 1. Endothelium – lining capillary wall is continuous with occluding junctions (impermeable) and is major structural component of parenchyma a. Basal lamina + pericytes are also part of capillary wall 2. Macrophages – in perivascular connective tissue may phagocytize antigenic molecules that escape lumen into parenchyma 3. Type I epithelioreticular cells – with occluding junctions provide protection to T cells, surround capillary wall in cortex Thymus is the site of T-cell Education – in fetus, thymus is populated by multipotent lymphoid stem cells from bone marrow destined to become T cells (Thymic cell education) -process is characterized by expression and deletion of CD antigens 1. Double-negative stage (early stage of differentiation) – expression of CD2 and CD7 on T cell surface; double negative meaning LACK of CD4 and CD8 2. Middle Stage – expression of CD1 3. Double-positive stage – T cells express TCR, CD3, CD4, and CD8; these cells are presented with self- and foreign antigens by type II/III epithelioreticular cells a. Positive Selection – if lymphocyte recognizes self-MHC molecule or foreign antigen, they survive – otherwise they will die b. Cells passing positive selection will migrate from cortex to medulla to undergo another selection process in where cells recognizing self-antigen by self-MHC are eliminated, called negative selection c. Cells surviving negative selection become either cytotoxic CD8+ T cells by losing CD4 or they become helper CD4+ T cells by losing CD8 d. This stage is called single-positive stage Spleen – size of fist; largest lymph organ in upper quadrant of abdominal cavity; rich vasculature -the spleen filters blood and reacts immunologically to blood-borne pathogens -spleen has both morphologic and immunologic filtering functions; has both large numbers of lymphocytes AND special vascular spaces or channels, a meshwork of reticular cells and fibers, and a large number macrophages and dendritic cells allowing spleen to monitor blood immunologically -spleen is enclosed by capsule from which trabeculae extend into parenchyma -trabeculae contain myofibroblasts producing CT fibers and can contract -spleen holds large volumes of RBCs in reserve; contraction of capsule and trabeculae helps discharge stored RBC into systemic circulation -Hilum – medial surface of spleen, is site of splenic artery, veins, nerves, and lymph vessels -lymph vessels originate in white pulp near trabeculaee and form a route for lymphocytes leaving spleen -spleen contains two regions white pulp and red pulp; white pulp is circular, surrounded by red pulp -white pulp – consists of thick accumulation of lymphocytes surrounding an artery, and is basophilic on H&E stains -splenic artery branches course through capsule/trabeculae and enter white pulp, becoming the central artery when inside -lymphocytes aggregate around central artery and constitute periarterial lymphatic sheath (PALS), which conforms to a cylindrical configuration down artery, and may resemble a lymphatic nodule -presence of central artery distinguishes PALS from other lymph nodules -nodules of PALS are B cell territory, wherase other lymphocytes of PALS are T cells surrounding the nodules -PALS may be considered thymus-dependent zone similar to deep cortex of lymph node -nodules contain germinal centers which develop as B cells proliferate after activation (24 hours after antigen exposure and may become enlarged -enlarged nodules are called splenic nodules Red Pulp contains large numbers of RBCs that it filters and degrades – red pulp consists of splenic sinuses separated by splenic cords (consist of loose meshwork of reticular cells and fibers that contain RBC, macrophages, lymphocytes, dendritic cells, plasma cells, and granulocytes) -splenic macrophages phagocytose damaged RBC; iron from RBC is used in formation of new RBC, and splenic macrophages begin process of hemoglobin breakdown Splenic or Venous Sinuses are Special Sinusoidal Vessels lined by Rod-Shaped Endothelial Cells – Endothelial cells lining splenic sinuses are extremely long running parallel to vessel -there are few contact points between adjacent cells, forming prominent intercellular spaces to allow RBC to pass in and out of sinuses -macrophages processes extend between endothelial cells into lumen of sinuses to monitor blood for antigens -sinuses do not have continuous basal lamina -Strands of Basal Lamina – loop around outside of sinus like hoops at right angles to long axis of endothelial cells -no smooth muscle or pericytes are at walls of splenic sinus Circulation within red pulp allows macrophages to screen antigens in blood – central artery sends branches to white pulp and to sinuses at perimeter of white pulp which are called marginal sinuses. -Central artery continues to red pulp where it branches into several arterioles called penicillar arterioles which continue as arterial capillaries -some of the capillaries are surrounded by aggregations of macrophages, making them called sheathed capillaries, which empty into reticular meshwork of splenic cords rather than connecting to endothelium-lined splenic sinuses -blood entering red pulp this way percolates through cords and is exposed to macrophages of cords before returning to circulation by squeezing through walls of splenic sinus -this is called open circulation and is the only route by which blood returns to venous circulation in humans (closed circulation happens in rat and dog) -open circulation exposes blood more efficiently to macrophages of red pulp -blood from sinuses drains to tributaries of trabecular veins that converge into lager veins and leaves spleen by splenic vein hepatic portal vein Spleen performs both immune and hemopoietic functions – spleen filters blood similar to lymph nodes and lymph, functions in both immune and hemopoietic systems -Immune systems include (all happen in white pulp): -antigen presentation by dendritic cells/macrophages and immune initiation -activation/proliferation of B and T cells -production of antibodies against antigen present in circulating blood -removal of macromolecule antigens from blood -Hemopoietic Functions of spleen (in red pulp) -remove and destroy senescent, damaged, abnormal RBC and platelets -retrieval of Fe from RBC hemoglobin -formation of RBC during early fetal life -storage of RBC in some species -role of red pulp is primarily blood filtration by removing particular material, macromolecular antigens, and aged, abnormal RBC and platelets -macrophages embedded in reticular meshwork of red pulp -senescent, damaged, abnormal RBC are broken down by lysosomes of macrophages and Fe of hemoglobin is retrieved and stored as ferritin or hemosiderin -hemo portion is broken down to bilirubin liver to form glucuronic acid and secreted into bile -Macrophages recognize senescent/abnormal blood cells in 2 ways: 1. Nonspecific mechanisms – morphologic and biochemical changes in aged erythrocytes cause them to be more rigid and easily trapped in mesh of red pulp 2. Specific mechanisms – opsonization of cell membrane with anti-band 3 IgG antibodies, which trigger Fc receptor-dependent phagocytosis of erythrocytes -also, specific glycosylation of glycophorins in aging erythrocytes act as recognition signal to trigger elimination -spleen is NOT essential for human life, and can be removed surgically, after which RBC destruction occurs in the bone marrow and liver