Prelab Questions 2012
advertisement

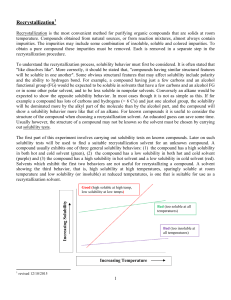
Pre-Lab Assignment #1 Student’s Name: __________________________________ 1. What are the 5 general steps in recrystallization. 5 pts 2. Assuming that sand, carbon, and salicylic acid are the main impurities in the sample of impure benzoic acid answer the following. You must use the solubility of these compounds in water in order to answer this question. You may use any reference text of URL but you must be prepared to give a proper citation for the source of the data. 6 pts Solubility of Sand in water _______ Citation _________________________________ Solubility of Carbon in water _______ Citation _________________________________ Solubility of Salicylic Acid in water ________ Citation ____________________________ 3. Other than inert, non-toxic, non-flammable, and inexpensive, what is the most important criteria for a recrystallization solvent? 1 pt 4. How will the insoluble impurities be removed from the impure benzoic acid? 1 pt 5. Why is the filter paper folded or fluted when doing a hot (gravity) filtration? 1 pt 6. Why is the funnel used for the hot filtration stemless? 1 pt 7. How will the soluble impurities be removed from the impure benzoic acid? 1 pt 8. After vacuum filtration, the solid remaining on the filter paper can either be rinsed with a small amount of cold, pure solvent to remove remaining “mother liquor”. What is “mother liquor” and why is it important to remove it? 1 pt 9. Why is it necessary to use a small amount of cold, pure solvent when rinsing with the pure solvent? 1 pt 10. Why should you break up all lumps before the final wash of the purified material on the filter paper? Explain. 2 pt