PPTB&W
advertisement

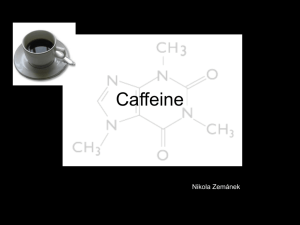
Isolation of Caffeine References: Slayden: pp. 47- 49 . Pavia: pp. 24, 73 – 84 Note: These pages are not in the GMU version of the Pavia text sold in the book, only in the primary text that we do not use anymore 1/17/2015 1 Isolation of Caffeine 1/17/2015 Overview An aqueous Vivarin/Sodium Carbonate mixture is extracted with Dichloromethane (Methylene Chloride) The organic fraction is separated from the aqueous solution and evaporated to dryness The product is recrystallized from Acetone The mass and % recovery of the product are determined Product identity is verified by Melting Point 2 Isolation of Caffeine Background Caffeine (a stimulant) is a bitter, white crystalline alkaloid derivative of Xanthine (note methyl substitution on primary amino groups) 1/17/2015 Caffeine is synthesized from the purine nucleotides AMP, GMP, and IMP in plants. These in turn are transformed into Xanthosine and then Theobromine, the latter being the penultimate precursor of Caffeine 3 Isolation of Caffeine The two Amide groups exist as zwitterionic resonance structures where the Nitrogen and Carbon atoms are double bonded to each other so that both of the Nitrogen atoms are essentially planer (in sp2 orbital hybridization) A zwitterion is a neutral molecule with a positive and a negative electrical charge (not dipoles) at different locations within that molecule Zwitterions are sometimes also called inner salts The fused ring system therefore contains a total of ten pi () electrons, thus, according to Huckel’s rule is aromatic 1/17/2015 4 Isolation of Caffeine Zwitterionic Resonance 1/17/2015 5 Isolation of Caffeine Chemical Properties 1/17/2015 Caffeine acts as a general nervous system stimulant, warding off drowsiness Caffeine is considered a “psycho active” substance, but is generally legal and unregulated Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, acting as a natural pesticide by paralyzing and killing insects Sources for human use include coffee plants, tea leaves, and kola nuts 6 Isolation of Caffeine Procedure Obtain 2 Vivarin tablets noting % of active ingredient Determine the mass of the tablets Obtain and determine mass of approximately 8 g of Sodium Carbonate Crush the tablets with a pestal & mortor Add the crushed sample to a 150 mL beaker Add 60 mL of Distilled Water Add the Sodium Carbonate (Na2CO3) to the solution Add a Teflon boiling chip Heat solution to boiling on a hot plate – maintain boiling point for 2 minutes 1/17/2015 7 Isolation of Caffeine 1/17/2015 Procedure (Cont’d) Allow the hot mixture to settle slightly - some solid particles will remain undissolved Attach an iron ring to a ring stand Insert a 125 ml Separatory Funnel into the iron ring using a clay triangle if necessary to support funnel Adjust height of iron ring so that stem of separatory funnel fits just inside a 150 ml beaker Place a glass funnel into the neck of the separatory funnel Make a boat of glass wool and place in the bottom of glass funnel 8 Isolation of Caffeine Procedure (con’t) 1/17/2015 Decant the sample solution into the glass funnel allowing the liquid to drain into the 125 mL Separatory Funnel Rinse the beaker with a small amount of distilled water and pass the water through the glass funnel to the separatory funnel Allow the filtrate in the Separatory Funnel to cool to room temperature 9 Isolation of Caffeine Procedure (con’t) 1/17/2015 Liquid–liquid Extraction, also known as solvent extraction and partitioning, is a method to separate two immiscible liquids, usually an aqueous component and an organic solvent component The process involves the extraction of a substance from one liquid phase into the other liquid phase utilizing a separatory funnel Use the following steps to extract the Caffeine from the aqueous phase into the organic phase consisting of Methylene Chloride solvent (density – 1.33 g/ml) Three (3) extractions will be performed 10 Isolation of Caffeine Procedure (Cont’d) Add 8.0 mL of Dichloroethane (Methylene Chloride) to the mixture in the separatory funnel Place the stopper in the top opening and make sure it is secure, i.e. won’t leak Invert the funnel pointing the stopcock end away from your face Use “gentle” swirling (avoids formation of an emulsion) for 10-15 seconds Slowly open the stopcock to relieve any pressure 1/17/2015 11 Isolation of Caffeine 1/17/2015 Close the stopcock and continuing swirling with venting every 15 seconds until no more gas is released (two or three times should be sufficient) Continue the swirling for a total of 5 minutes Replace the separatory funnel in the ring stand and allow the mixture to settle for a few minutes Remove the stopper from the Separatory Funnel Open the stopcock slowly and drain the clear bottom (organic) layer into a clean 150 mL beaker Repeat the extraction process two more times with new 8.0 mL aliquots of Methylene Chloride, but reduce the total swirling time to 2 minutes Each time, add the clear bottom layer to the 150 mL beaker 12 Isolation of Caffeine Procedure (Cont’d) With the help of the instructor, add Anhydrous Sodium Sulfate as a drying agent Leaving the particles settled on the bottom of the beaker, slowly pour (decant) the liquid into a clean, dry, 50 mL beaker Rinse the beaker containing the drying agent particles on the bottom with one additional small portion of Dichloromethane and add to the sample beaker Evaporate the mixture to dryness in the hood Note: Set hot plate setting to about 3 Note: Crude yield at this point is roughly 90% 1/17/2015 13 Isolation of Caffeine Procedure (Cont’d) Recrystallization Process using a mixed solvent of Acetone and Petroleum Ether Dissolve the dried product in 12-15 mL cold Acetone (from ice/water bath) Heat solution gently on hot plate If solution is incomplete, remove beaker from hot plate and add more Acetone in 2 mL increments Up to 20 mL of additional Acetone may be required until a clear pale-yellow solution is obtained Remove solution from hot plate and allow to cool slightly 1/17/2015 14 Isolation of Caffeine Procedure (Cont’d) Add Petroleum Ether, drop by drop, with swirling from a full plastic pipet until the first appearance of pervasive cloudiness throughout the solution Continue cooling until crystallization is complete Note: Maximum yield is obtained when the addition of Petroleum Ether is stopped at the first appearance of a pervasive cloudiness throughout the solution 1/17/2015 It may be necessary to boil off a portion of the solution to effect recrystallization Separate the crystals from the solution by Vacuum Filtration rinsing the beaker and crystals in the funnel with small amounts of Petroleum Ether 15 Isolation of Caffeine Immerse beaker in an ice-water bath for about a minute Air-dry the sample from the evaporation procedure or the vacuum filtration procedure on a pre-weighed weighing tray for one week Compute the mass of the purified sample Compute the percent recovery of the product relative to the amount of caffeine indicated in the tablets Determine melting point - 236 oC Note: Decomposition of this crude material occurs beginning at 236oC over a range of 5oC or so 1/17/2015 16