Vapor Pressure - Hudson City Schools
advertisement

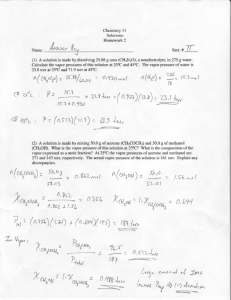
Vapor Pressure Vapor Pressure Pressure of the vapor present when equilibrium is achieved between the rate of vaporization and the rate of condensation. At the boiling point, the Patm = Pvapor As the vapor pressure on a pot of water is reduced, the energy needed to boil that water is also reduced. Pressure Cooker: By increasing the vapor pressure, additional energy is needed for the water to boil, therefore the water can boil at temperatures above 100C. Effect of Pressure on Boiling Point Vapor Pressure vs. Temperature As the temperature increases, a greater number of molecules have sufficient kinetic energy to convert from the liquid to the vapor phase. There is a nonlinear relationship between the vapor pressure of a liquid and temperature. Vapor Pressure vs. Temperature The Clausius – Clapeyron Equation • A mathematical expression which relates the variation of vapor pressure to temperature • ln P = (-DHvap/RT) + C where C is a constant • IMPORTANCE: • When the ln P is plotted vs (1/T) you create a line where the slope is equal to the –DHvap/R • Which means you can calculate the enthalpy of vaporization from the slope of the line. • R = 8.314 J/Kmol Convert all Temps to Kelvin Vapor Pressure of Solutions • A nonvolatile solute lowers the vapor pressure of the solution. • The molecules of the solvent must overcome the force of both the other solvent molecules and the solute molecules. Raoult’s Law: •Psoln = csolvent x Psolvent • Vapor pressure of the solution = m ole fraction of solvent x vapor pressure of the pure solvent • Applies only to an ideal solution where the solute doesn’t contribute to the vapor pressure. • Water has a higher vapor pressure than a solution Aqueous Solution Pure water • Water evaporates faster from for water than solution Aqueous Solution Pure water • The water condenses faster in the solution so it should all end up there. Aqueous Solution Pure water Practice Problem • A solution of cyclopentane with a nonvolatile compound has vapor pressure of 211 torr. If vapor pressure of the pure liquid is 313 torr, what is the mole fraction of the cyclopentane? • Psoln = XcpPcp • 211 torr = Xcp (313 torr) • .674 Try one on your own • Determine the vapor pressure of a solution at 25 C that has 45 grams of C6H12O6, glucose, dissolved in 72 grams of H2O. The vapor pressure of pure water at 25 C is 23.8 torr. • Psolution= Xsolvent Psolvent • Psolution = .941(23.8 torr) • Psolution = 22.4 torr Ideal solutions • Liquid-liquid solutions where both are volatile. • Modify Raoult’s Law to • Ptotal = PA + PB = cAPA0 + cBPB0 • Ptotal = vapor pressure of mixture • If this equation works then the solution is ideal. Vapor Pressure of solution Deviations • • • • If solvent has a strong affinity for solute (H bonding). Lowers solvent’s ability to escape. Lower vapor pressure than expected. Negative deviation from Raoult’s law. • DHsoln is large and negative exothermic. • Endothermic DHsoln indicates positive deviation. Vapor Pressure Positive deviationsWeak attraction between solute and solvent Positive ΔHsoln χA χb Vapor Pressure Negative deviationsStrong attraction between solute and solvent Negative ΔHsoln χA χb Problem #1 • The vapor pressure of a solution containing 53.6g of glycerin C3H8O3 in133.7g ethanol C2H5OH is 113 torr at 40C. Calculate the vapor pressure of pure ethanol at 40C assuming that the glycerin is a non volatile, nonelectrolyte solute in ethanol. Answer to #1 Psoln = Xeth Peth 113torr = 2.90mol/3.48mol (Peth) 135.6 torr = Peth Problem #2 • At a certain temperature, the vapor pressure of pure benzene C6H6 is 0.930atm. A solution was prepared by dissolving 10.0g of a nondissociating, nonvolatile solute in 78.11g of benzene at that temperature. The vapor pressure was found to be 0.900atm. Assuming the solution behave ideally, determine the molar mass of the solute. Answer #2 Psoln = XbenzenePbenzene .900atm = Xbenzene (.930atm) Xbenzene = .9677 Xsolute = .0323 MM = 10.0g/.0323mol = 310g/mol Problem #3 A solution of NaCl in water has a vapor pressure of 19.6 torr at 25C. What is the mol fraction of solute particle in this solution if the vapor pressure of water is 23.8 torr at 25C? Answer #3 Psoln = XwaterPwater 19.6torr = Xwater(23.8torr) .824 = Xwater therefore Xsolute = .176 Problem #3 For the same problem as #3: What is the vapor pressure of the solution at 45C if the vapor pressure of water is 71.9 torr at 45C? Answer #3 Psoln = .824(71.9torr) Psoln = 59.2 torr Problem #4 A solution is made from 0.0300mol CH2Cl2 and 0.0500mol CH2Br2 at 25C. Assuming that the solution is ideal, calculate the % composition of the vapor at 25C. PCH2Cl2 = 133 torr PCH2Br2 = 11.4 torr Answer #4 Psoln = XCH2Cl2 P + XCH2Br2 P Psoln = (.03/.08)(133torr) + (.05/.08)(11.4 torr) Psoln = 57.0 torr XCH2Cl2 = 49.9 / 57 = .875 = 87.5% XCH2Br2 = 7.13 / 57 = .125 = 12.5%