Hein and Arena - faculty at Chemeketa
advertisement

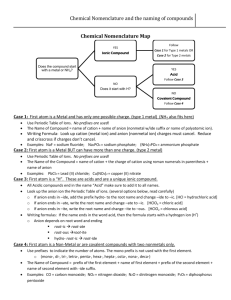
1 Common and Systematic Names 2 Chemical nomenclature is the system of names that chemists use to identify compounds. Two classes of names exist: common names and systematic names. 3 • Common names are arbitrary names. – They are not based on the composition of the compound. – They are based on an outstanding chemical or physical property. • Chemists prefer systematic names. – Systematic names precisely identify the chemical composition of the compound. – The present system of inorganic chemical nomenclature was devised by the International Union of Pure and Applied Chemistry (IUPAC). 4 5 Elements and Ions 6 The formula for most elements is the symbol of the element. Sodium Potassium Zinc Argon Mercury Lead Calcium Na K Zn Ar Hg Pb Ca 7 These 7 elements are found in nature as diatomic molecules. Hydrogen Nitrogen Oxygen Fluorine Chlorine Bromine Iodine H2 N2 O2 F2 Cl2 Br2 I2 8 Ions 9 If charged A one orparticle moreknown electrons as an ion are can be produced removed from abyneutral adding or atom a removingion positive one is or formed. more electrons A positive ion from is called a neutral a cation. atom. remove e- → neutral atom 10 Positive Ion Formation: Loss of Electrons From a Neutral Atom Na Na+ + e- Ca Ca2+ + 2eAl Al3+ + 3e- 11 Naming Cations 12 Cations are named the same as their parent atoms 13 Atom Cation Name of Cation sodium (Na) + Na sodium ion 14 Atom Cation Name of Cation calcium (Ca) 2+ Ca calcium ion 15 Atom Cation Name of Cation lithium (Li) + Li lithium ion 16 Atom Cation Name of Cation magnesium (Mg) 2+ Mg magnesium ion 17 Atom Cation Name of Cation strontium (Sr) 2+ Sr strontium ion 18 A If charged one or more particle electrons known as areanadded ion can to abe neutral produced atombyaadding negative or ion is removing formed. Aone negative or more ionelectrons is called an from anion. a neutral atom. add e- neutral atom → 19 Naming Anions 20 An anion consisting of one element has the stem of the parent element and an – ide ending 21 Atom Anion fluorine (F) F stem Name of Anion fluoride ion 22 Atom Anion chlorine (Cl) Cl stem Name of Anion chloride ion 23 Atom Anion bromine (Br) Br stem Name of Anion bromide ion 24 Atom Anion nitrogen (N) 3N stem Name of Anion nitride ion 25 Atom Anion phosphorous (P) 3P stem Name of Anion phosphide ion 26 Atom Anion oxygen (O) 2O stem Name of Anion oxide ion 27 Binary Compounds 38 Binary compounds contain only two different elements. 39 Binary ionic compounds consist of a metal combined with a non-metal. 40 A. Binary Ionic Compounds Containing a Metal Forming Only One Type of Cation (one charge) 41 Type I Cations include: • • • • the Group A metals Hydrogen B metals with one charge: Zn+2, Cd+2, Ag+ The polyatomic ion NH4+ 42 • The chemical name is composed of the name of the metal followed by the name of the nonmetal which has been modified to an identifying stem plus the suffix –ide. • Using this system the number of atoms of each element present is not expressed in the name. 43 Name of Metal + Stem of Nonmetal plus -ide ending 44 (ur) 45 If hydrogen is written first in the formula, it is treated as if it were a group IA metal. 46 Name the Compound CaF2 Step 1 From the formula it is a two-element compound and follows the rules for binary compounds. 47 Name the Compound CaF2 Step 2 The compound is composed of Ca, a metal and F, a nonmetal. Ca forms only a +2 cation. Thus, call the positive part of the compound calcium. 48 Name the Compound CaF2 Step 3 Modify the name of the second element to the stem fluor- and add the binary ending –ide to form the name of the negative part, fluoride. 49 Name the Compound CaF2 Step 4 The name of the compound is therefore calcium fluoride. 50 Examples 51 Compound NaCl name of metal Name sodium chloride nonmetal stem 52 Compound HCl(g) name of metal Name For naming purposes only, hydrogen is treated as if it were a group IA metal. hydrogen chloride nonmetal stem 53 Compound MgCl2 name of metal Name magnesium chloride nonmetal stem 54 Compound K2O name of metal Name potassium oxide nonmetal stem 55 Compound Na3P name of metal Name sodium phosphide nonmetal stem 56 B. Binary Ionic Compounds Containing a Metal That Can Form Two or More Types of Cations (two or more charges) 57 Type II Cations include: • B metals with two charges • Zn+2, Cd+2, Ag+ are excluded 58 Name the Compound FeS Step 1 This compound follows the rules for a binary compound. 59 Name the Compound FeS Step 2 ItInissulfides, a compound the of Fe, aon charge metal, S is –2. and S, a nonmetal, the Therefore andcharge Fe is a on transition Fe must bemetal +2, and thatthe has more than name of theone positive type of cation. part of the compound is iron (II).(or ferrous) 60 Name the Compound FeS Step 3 We have already determined that the name of the negative part of the compound will be sulfide. 61 Name the Compound FeS Step 4 The name of FeS is iron(II) sulfide.(or ferrous sulfide) 62 The IUPAC (Stock) System 63 The metals in the center of the periodic table (including the transition metals) often form more than one type of cation. 64 Each ion of iron forms a different compound with the same anion. Fe2+ FeS Fe3+ Fe2S3 65 In IUPAC the IUPAC devised System the Stock the charge System on the of cation is designated nomenclature by to a name Roman compounds numeral placed of in parentheses metals thatimmediately have more than following one type theofname of the cation. metal. Cation Charge +1 +2 +3 +4 +5 Roman Numeral I II III IV V The nonmetal name ends in -ide. 66 Stock System IUPAC System Lower Lower Charge Charge Element Formula Higher HigherCharge Charge Name Formula Name Copper Cu+ copper(I) Cu2+ copper(II) Iron Fe2+ iron(II) Fe3+ iron(III) Lead Pb2+ lead(II) Pb4+ lead(IV) Mercury Hg22+ mercury(I) Hg2+ mercury(II) Tin Sn2+ tin(II) Sn4+ tin(IV)67 Examples 68 iron(II) chloride FeCl2 +2 iron(II) -1 chloride compound ion ioncharge name name iron(III) chloride FeCl3 +3 iron(III) -1 chloride 69 tin(II) bromide SnBr2 +2 tin(II) -1 bromide compound ion ioncharge name name tin(IV) bromide SnBr4 +4 tin(IV) -1 bromide 70 The Classical System 71 In the Classical System the name of the metal (usually the Latin name) is modified with the suffixes -ous and ic. 72 Metal name ends in -ous lower charge -ic higher charge nonmetal name ends in -ide 73 Examples 74 ferrous chloride FeCl2 +2 ferrous -1 chloride ion compound ioncharge name name ferric chloride FeCl3 +3 ferric -1 chloride 75 stannous bromide SnBr2 +2 stannous -1 bromide compound ion ioncharge name name stannic bromide SnBr4 +4 stannic -1 bromide 76 Ion Names: Classical System Lower Charge Higher Charge Element Formula Name Formula Name Copper Cu+ cuprous Cu2+ cupric Iron Fe2+ ferrous Fe3+ ferric Lead Pb2+ plumbous Pb4+ plumbic Mercury Hg22+ mercurous Hg2+ mercuric Tin Sn2+ stannous Sn4+ stannic 77 Binary Compounds Containing Two Nonmetals 78 Compounds between nonmetals are molecular, not ionic. 79 In a compound formed between two nonmetals, the element that occurs first in this series is named first. • • • • • • Si B P H C S • • • • • • I Br N Cl O F 80 Prefixes 81 A Greek prefix is placed before the name of each element to indicate the number of atoms of the element that are present. 82 Mono is rarely used when naming the first element. • mono = 1 • di = 2 • tri = 3 • tetra = 4 • penta = 5 • • • • • hexa = 6 hepta = 7 octa = 8 nona = 9 deca = 10 83 Examples 84 dinitrogen trioxide N2 O 3 indicates two nitrogen atoms indicates three oxygen atoms 85 phosphorous pentachloride PCl5 indicates one phosphorous atom indicates five chlorine atoms 86 dichlorine heptaoxide Cl2O7 indicates two chlorine atoms indicates seven oxygen atoms 87 Determine the Name of PCl5 Step 1 • There are 2 elements present. • The compound is binary. • Phosphorous and chlorine are nonmetals so the rules for naming binary compounds of 2 nonmetals apply. • Phosphorous is named first. Therefore the compound is a chloride. 88 Determine the Name of PCl5 Step 2 • No prefix is needed for phosphorous because each molecule of PCl5 has only one phosphorous atom. The prefix penta- is used with chloride because there are 5 chlorine atoms present in one molecule. Step 3 • The name is phosphorous pentachloride. 89 Examples 90 Cl2O3 dichlorine trioxide 91 N2O3 dinitrogen trioxide 92 CCl4 carbon tetrachloride 93 CO carbon monoxide 94 Name CO2 carbon dioxide 95 Name PI3 phosphorous triiodide 96 D. Acids Derived from Binary Compounds 97 • Certain binary hydrogen compounds, when dissolved in water, form solutions that have acid properties. • The aqueous solutions of these compounds are given acid names. • The acid names are in addition to their –ide names. • Hydrogen is typically the first element of a binary acid formula. 98 Acid Formation binary hydrogen compound (not an acid). water acid 99 Pure compound HCl -ide Dissolved in water HCl acid 100 • To name binary acids write the symbol of hydrogen first. • After hydrogen write the symbol of the second element. • Place the prefix hydro- in front of the stem of the nonmetal name. • Place the suffix -ic after the stem of the nonmetal name. 101 Examples 102 Pure Compound HCl (g) hydrogen chloride 103 Dissolved in Water HCl (aq) hydrochloric acid 104 Pure Compound HI (g) hydrogen iodide 105 Dissolved in Water HI (aq) hydroiodic acid 106 Pure Compound H2S (g) hydrogen sulfide 107 Dissolved in Water H2S (aq) hydrosulfuric acid 108 Pure Compound H2Se (g) hydrogen selenide 109 Dissolved in Water H2Se (aq) hydroselenic acid 110 111 Naming Compounds Containing Polyatomic Ions 112 A polyatomic ion is an ion that contains two or more elements. NO 3 113 • Compounds containing polyatomic ions are composed of three or more elements. • They usually consist of one or more cations combined with a negative polyatomic ion. Na 2CO3 114 • When naming a compound containing a polyatomic ion, name the cation first and then name the anion. Na 2CO3 115 This is the way the formula is written. KMnO 4 K + MnO 4 The ions are what is actually present. 116 This is the way the formula is written. Na 2CO3 2Na + CO 23 The ions are what is actually present. 117 Prefixes and Suffixes Elements that Form More than One Polyatomic Ion with Oxygen 118 Anions ending in -ate always contain more oxygen than ions ending in -ite. nitrite 2 NO nitrate 3 NO 119 Anions ending in -ate always contain more oxygen than ions ending in -ite. phosphite 33 PO phosphate 34 PO 120 Anions ending in -ate always contain more oxygen than ions ending in -ite. sulfite 23 SO sulfate 24 SO -ate and –ite do not indicate the number of oxygen atoms. 121 per- (short form of hyper) denotes anions with more oxygen than the -ate form . chlorate 3 ClO perchlorate 4 ClO 122 hypo- denotes anions with less oxygen than the -ite form. hypochlorite - ClO chlorite 2 ClO 123 Oxy-Anions and Oxy-Acids of Chlorine (also Bromine and Iodine) Anion Anion Name Acid Acid Name ClO– hypochlorite HClO hypochlorous acid ClO2– chlorite HClO2 ClO3– chlorate HClO3 chlorous acid chloric acid ClO4– perchlorate HClO4 perchloric acid 124 Four ions do not use the –ate/ite system. hydroxide - cyanide - hydrogen sulfide - peroxide 22 OH HS CN O 125 There are three common positively charged polyatomic ions. mercury(I) 2+ 2 Hg hydronium + 3 HO ammonium + 4 NH 126 127 Names of Selected Compounds That Contain More Than One Kind of Positive Ion Formula Name of compound KHSO4 potassium hydrogen sulfate Ca(HSO3)2 calcium hydrogen sulfite NH4HS ammonium hydrogen sulfide MgNH4PO4 magnesium ammonium phosphate NaH2PO4 sodium dihydrogen phosphate Na2HPO4 disodium hydrogen phosphate KHC2O4 potassium hydrogen oxalate KAl(SO4)2 potassium aluminum sulfate Al(HCO3)3 aluminum hydrogen carbonate 128 Acids 129 Oxy-acids contain hydrogen, oxygen and one other element. • The other element is usually a nonmetal, but it can be a metal. • Its first element is hydrogen. • Its remaining elements include oxygen and form a polyatomic ion. 130 Hydrogen in an oxy-acid is not expressed in the acid name. The word acid in the name indicates the presence of hydrogen. 131 indicates hydrogen sulfuric acid contains contains contains hydrogen sulfur oxygen H 2SO 4 132 Anions ending in -ate always contain more oxygen than ions ending in -ite. phosphite 33 PO phosphate 34 PO 133 Naming the Acid Based on the Name of the Polyatomic Ion Ending of Polyatomic Ion ite Ending of Acid ous less oxygen ate ic more oxygen 134 Examples 135 sulfite SO 2 3 sulfurous acid H 2SO3 136 sulfate SO 2 4 sulfuric acid H 2SO 4 137 nitrite NO 2 nitrous acid HNO2 138 3 nitrate NO nitric acid HNO3 139 140 141 142