Matter and Changes
advertisement

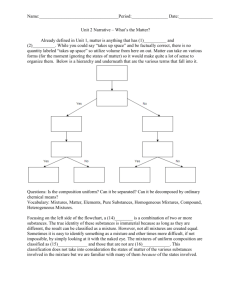
July 2004 Chemistry: the science that deals with the composition, structure and behavior of matter. It is also concerned with the energy and the energy changes associated with matter. Matter • Volume is the amount of three dimensional space an object occupies. • Mass is a measure of the amount of matter. It is a function of the amount of atoms in a substance. • Matter is anything that has mass and takes up space. CLASSIFICATION OF MATTER ACCORDING TO ITS STATE Solid, liquid and gas. STATES OF MATTER Matter Gas Liquid Solid STATES OF MATTER Matter Gas Liquid Variable shape and volume that fill container. Variable shape: It adopts the shape of the container. Random particle movement. Definite volume. Large intermolecular distances. Random particle movement within fixed volume. Particles are closer than in the gas state. Solid Definite shape and volume. Restricted particle movement to fixed positions: vibration. Short intermolecular distances. Crystalline and amorphous structures. Plasma – at extreme temperatures like those of the sun, high-energy collisions between atoms may strip off electrons, creating plasma, in which positive nuclei move about in a sea of electrons. Stars are composed of a dense hydrogenand helium-rich plasma mixture. Properties of Matter Physical properties: properties which can be observed without changing the composition of matter. Physical Change Solid iodine is heated and it sublimes, that is it changes from a solid to a gas state directly. CHANGES IN THE STATES OF MATTER MELTING -The process of a solid transforming to a liquid FREEZING - The process of converting a liquid into a solid. CONDENSATION - the gas being converted to a liquid. EVAPORATION - the liquid being converted to a gas. Freezing Melting Melting Condensation Evaporation Chemical properties: describe the ability of a substance to form a new substance. They are only observed when the substance undergoes a change in composition. CHEMICAL PROPERTIES For example, hydrogen has the potential to ignite and explode given the right conditions. This is a chemical property. Metals in general have they chemical property of reacting with an acid. Zinc reacts with hydrochloric acid to produce hydrogen gas. This is a chemical property. See video for differences between physical and chemical properties. See video for differences between physical and chemical changes. Chapter 1 Chemical Reactions • The reactants are the substances that react in a chemical change. • The products are the substances that are formed by the chemical change. reactants product Carbon plus oxygen yields (or forms) carbon dioxide. carbon + oxygen carbon dioxide • Evidence of a Chemical Change Evolution of a gas Formation of a precipitate Energy changes light, sparks, warm or cold container Color Change Properties of matter Physical properties Chemical properties Can be observed without changing the substance into another substance. Describes the way the substance undergoes or resists change to form another substance. Color Odor Texture Boiling point Melting point Density Solubility Decomposition Explosiveness Flammability Combustibility Corrosion Reactivity Classification of changes as physical or chemical Change Rusting of iron Melting of snow Sharpening a pencil Digesting food Taking a bite of food Burning gasoline Slicing an onion Detonation of dynamite Souring of milk Breaking of glass Classification Chemical Physical Physical Chemical Physical Chemical Physical Chemical Chemical Physical Classification of Matter Mixtures 1. Physical combination of two or more substances. 2. Variable composition. 3. Properties vary as composition varies. 4. Components can be separated using physical means. Pure Substances 1.Only one substance is present. 2. Definite and constant composition. 3. Properties are always the same under a given set of conditions. Classification of Matter Mixtures Heterogeneous Pure Substances Homogeneous 1. Two or more distinct phases. 2. Each phase has different properties 1. Only one visibly distinct phase. 2. The phase has the same properties throughout. Classification of Matter Mixtures Pure Substances Compounds 1. A chemical combination of two or more elements. 2. Can be broken down into constituent elements using chemical, but not physical means. 3. Has a definite, constant elemental composition. See video Elements 1. Cannot be broken down into simpler substances by chemical or physical means. 2. The building blocks for all other types of matter. 3. 115 elements are known. Homogeneous Heterogeneous Physical methods to separate mixtures Filtration - method to separate a liquid from a solid. Physical methods to separate mixtures Distillation – to separate mixtures by using the differences in boiling point. See periodic table video 1A 1 H 1.008 3 Li 6.941 11 The symbols of the elements in red are derived from Latin Na natrium Sn stannum K kalium Sb stibium 2A 3A 4A 5A Fe ferrum Au aurum 4 5 6 7 Cu cuprum Hg hydrargyrum Be B C N 9.012 10.811 12.011 14.007 Ag argentum Pb plumbum 12 Na Mg 22.99 24.305 13 8B 3B 4B 5B 6B 7B 1B 2B 14 8A 2 He 6A 7A 4.0026 8 9 10 O F Ne 15.999 18.998 20.179 16 17 18 15 Al Si P S Cl Ar 26.981 28.086 30.974 32.066 35.453 39.948 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.098 40.078 44.956 47.88 50.94 51.996 54.938 55.847 58.93 58.69 63.546 65.39 69.723 72.61 74.922 78.96 79.904 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 85.468 131.29 88.906 91.224 92.906 95.94 (98) 101.07 102.9 106.4 107.87 112.41 114.82 118.71 121.75 127.60 126.9 131.29 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 132.9 137.33 178.49 180.95 183.85 186.2 190.2 192.2 195.1 196.97 200.59 204.38 207.2 208.98 (209) (210) (222) 87 88 104 105 106 107 108 109 110 111 112 114 Fr Ra Rf Db Sg Bh Hs Mt Uun Uuu Uub Uug (223) (226) (261) (262) (266) (264) (277) (268) (272) (272) (285) (289) The symbol of the element in blue is derived from German 57 58 59 60 61 62 63 64 65 66 67 W 68 wolfram 69 70 71 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 138.9 140.1 140.9 144.24 (145) 150.36 151.97 157.25 158.96 162.5 164.93 167.26 168.93 173.04 174.97 89 90 91 92 93 94 95 96 97 98 99 100 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No 103 Lr (227) 232.04 231.04 238.03 (237) (244) (243) (247) (247) (251) (252) (257) (258) (259) (260) Basic Laws of Matter Law of Conservation of Mass - first published by Antoine Lavoisier, French chemist (1743-1794). It states that when a chemical or physical change occurs, the total mass after the change is the same as the total mass before the change. Mass: is independent from the location. Weight: the measure of the earth’s gravitational attraction for a body. In general, it is a measure of the gravitational force between two masses. Balance - The instrument used in the lab to get the mass of an object. Scale - The instrument used in the lab to get the weight of an object. Mostly used in Physics Some common laboratory glassware for measuring volume.