Chapter 18 Study Guide Answers
advertisement

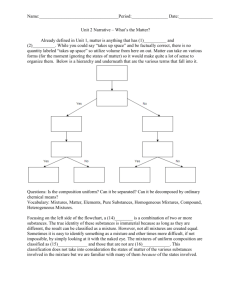
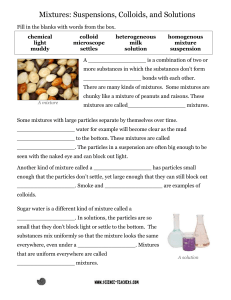
Physical Science – Chapter 18 Study Guide Answers Chapter Objectives 1. Define pure substances and mixtures. Pure substances – type of matter with a fixed composition, element or compound (composition is definite) Mixtures – 2 or more substances that can be separated by physical means (Composition is variable) 2. Identify elements and compounds. Elements – pure substances in which all atoms have the same identity Compounds – when 2 or more different elements combine in a fixed proportion 3. Compare and contrast solutions, colloids, and suspensions. Solutions – particles are so small they cannot be seen with a microscope and will never settle out; light cannot pass through, homogenous mixtures Colloids – particles that are larger than those in solution but not heavy enough to settle out; Ex: milk, light passes through/scatters (Tyndall effect) Suspensions – heterogeneous mixtures, contains liquid, particles will settle if left undisturbed, Ex: pond water; will scatter light 4. Describe the phase and energy changes associated with STATE changes. Changing states of matter (from solids, liquids, or gas to another state), physical changes Melting – solid to a liquid, add energy Freezing: liquid to solid, releases energy Vaporization: liquid to gas, add energy Boiling: throughout the liquid Evaporation: surface only Condensation: gas to liquid, releases energy Sublimation: solid to gas, adds energy 5. Identify substances using physical properties. Physical properties – properties than can be observed without altering identity of substance Alterations in form but not identity Density, freezing/melting point, color 6. Compare and contrast physical and chemical changes. Physical changes – doesn’t change the identity, sometimes it is reversible Tear, folding, dissolving, grind, phase change Chemical changes – changes the identity, produces something new, not reversible Burn, release a gas 7. Identify chemical changes. Burn (flammability), Rust, Decay, Digest, Corrosion 1) Color change 2) Odor change 3) Temperature change 4) Bubbles produced (gas released) 5) Precipitate (new solid produced) 8. Determine how the law of conservation of mass applies to chemical changes. Law = mass cannot be created nor destroy Mass you start with is the same as you end Key Concepts 9. Types of Physical Changes (give examples) See #5, tear, break, grind, dissolve, bursting a balloon, crumpling paper 10. Types of Chemical Changes (give examples) See #7, color/dye your hair, release gases, eat & digest food, when limestone reacts with acid rain, cooking 11. Homogenous mixtures 2 or more substances blended evenly throughout (Cannot see the different parts) Ex: soft drinks, solutions 12. Heterogeneous mixtures Mixture in which different materials can be easily distinguished Ex: pasta salad, fruit salad, concrete, granite 13. Tyndall effect Scattering of light by colloid particles 14. Melting point & Freezing Point Water’s melting/freezing point = 0C or 32F Same temperature 15. Heat of fusion the amount of energy required to change 1 kg of a substance from a solid to a liquid at its melting point or released when a liquid is changed to a solid at its freezing point 16. Heat of vaporization the amount of energy required for 1 kg of the liquid at its boiling point to become a gas; energy released during condensation when a gas becomes a liquid 17. Three types of matter Solid, gas, liquid (4th = plasma)