Thermochemistry Study Guide: Equations, Heat, Hess's Law
advertisement

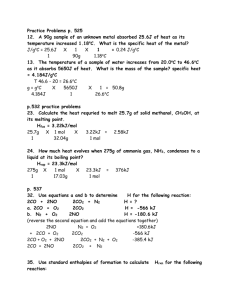
Name ____________________________________ Date ________ Period ____ Chapter 17 Thermochemistry Study Guide 17.1 – 17.2 Thermochemical Equations 1. Make the following conversions: a. 444 cal to joules = 1.86 x 103 J b. 1.8 kJ to joules = 1.8 x 103 J c. 0.45 kJ to calories = 1.1 x 102 cal 2. Classify each of these processes as endothermic or exothermic: a. condensing steam – exo c. evaporating alcohol – endo b. burning alcohol – exo d. baking a potato – endo 3. The specific heat capacity for silver is 0.24 J/goC. Calculate the energy required to raise the temperature of 150.0 g Ag from 273 K to 298 K. Calculate the molar heat capacity of silver. Energy: q = (150.0)(.24)(298-273) = 9.0 x 102 J Molar heat capacity: (0.24 J/goC)(107.87 g/mol Ag) = 26 J/mol 4. It takes 585 J of energy to raise the temperature of 125.6 g Hg from 20.0oC to 53.5oC. Calculate the specific heat capacity and the molar heat capacity of Hg. Specific heat capacity: C = q/mΔt = 585/(125.6)(53.5-20) = 0.139 J/goC Molar heat capacity: (0.139 J/goC)(200.59 g/mol Hg) = 27.9 J/mol 5. A 46.2-g sample of copper is heated to 95.4oC and then placed in a calorimeter containing 75.0 g water at 19.6oC. The equilibrium temperature in the calorimeter is 21.8oC. Calculate the specific heat capacity of copper; assuming that all the heat lost by the copper is gained by the water. C = 0.203 J/goC 6. A 15.0-g sample of nickel metal is heated to 100.0oC and dropped into 55.0 g of water, initially at 23.0oC. Assuming that all the heat lost by the nickel is absorbed by the water; calculate the final temperature of the nickel and the water. The specific heat of nickel is 0.444 J/goC. 15.0(.444)(100.0 – Tf) 666 – 6.66Tf 5958.76 25.2oC = 55.0(4.184)(Tf – 23.0) = 230.12Tf – 5292.76 = 236.78 Tf = Tf Name ____________________________________ Date ________ Period ____ 7. Haley was running bath water and realized it was too hot. If she has 20.0 L of water in the tub at 95oC and then adds 15.8 L of water at 75oC, what will the final temperature of the water be? 20(95 – Tf) 1900 – 20Tf 3085 86.2 oC = 15.8(Tf – 75) = 15.8 Tf – 1185 = 35.8 Tf = Tf 8. For the combustion of methane, calculate the enthalpy change for the burning of 1.00 g of methane. CH4(g)+ 2O2(g) CO2(g) + 2H2O(l) + 890 kJ 1molCH 4 890kJ H 1.00 gCH 4 16.05gCH 4 1molCH 4 ΔH = - 55.5 kJ 17.3 Heat in Changes of State 9. Read heating curves (See Changes of State w/s) Label the phases, boiling point and melting point here: boiling point = 20oC , melting point = 10oC a = solid b = melting c = liquid d = boiling e = gas Indicate whether a heating curve would be flat or rising in 10-14. 10. liquid is boiling flat 11. solid is warming – rising 12. solid is melting – flat 13. PE is increasing – flat 14. KE is increasing – rising Heat of Fusion and Heat of Vaporization Problems Values for Water: (SHOW YOUR WORK FOR FULL CREDIT!!) Hfus = 6.009 kJ/mol Hvap = 40.79 kJ/mol o o CH2O(s) = 2.108 J/g C CH2O(l) = 4.184 J/g C CH2O(g) = 1.87 J/goC 15. How much heat needs to be added to 10090 grams of ice at -10.0oC so that it all becomes a vapor at 120.0oC? A q = mCΔT = (10090g)(2.108 J/goC)(0-(-10.0)) = 212697.2 J Name ____________________________________ Date ________ Period ____ B ΔH = C q = mCΔT = (10090g)(4.184 J/goC)(100-0) = 4221656 J D ΔH = 10090 g H 2 O 1 mol H 2 O 6.009kJ 1000 J = 3364639.8 J 1 18.02 g H 2 O 1 mol H 2 O 1kJ 10090 g H 2 O 1 mol H 2 O 40.79kJ 1000 J = 22839683.7 J 1 18.02 g H 2 O 1 mol H 2 O 1kJ E q = mCΔT = (10090g)(1.87 J/goC)(120-100) = 377366 J A + B + C + D + E = 31016042.7 J = 3.10 x 107 J 16. If you are freezing special green ice cubes for St. Patrick’s Day, how much energy do you need to remove from 2 ice trays of 12 cubes if each cube holds 24.2 grams of water at 0oC? 194 kJ 17. Trevor has a cold and wants to make steam in a vaporizer to help clear his congestion. How much energy will be required to change one gallon of water at 100oC to steam at 100oC? (The density of water is 1.00g/ml) 8566 kJ (no sig figs specified) 18. Rachel’s prom dress is all wrinkled, but she doesn’t have the time or patience to iron it. How much energy will be required to fire up the steamer to remove wrinkles if she add 521 grams of water at 25.0oC and changes it all to steam at 100.oC? C q = mCΔT = (521g)(4.184 J/goC)(100-25) = 163489.8 J D ΔH = 521g H 2 O 1 mol H 2 O 40.79kJ 1000 J = 1179333.5 J 1 18.02 g H 2 O 1 mol H 2 O 1kJ C + D = 1342823.3 J = 1.34 x 106 J 19. The steamer Andrew is using to strip the NASCAR wallpaper off his bedroom walls has used 32,967 kJ of energy. How many grams of water at 100oC has he changed to steam at 100oC? 14564 kJ 20. Using a coffee cup calorimeter, Abby finds a balance that works and adds 1.60 g of NH4NO3 to 75.0 g of water at an initial temperature of 25.00oC. Name ____________________________________ Date ________ Period ____ After the salt completely dissolves, the final temperature of the solution is 23.34oC. Assuming no heat is lost to the calorimeter; calculate the enthalpy change for the dissolution of NH4NO3 in kJ/mol. ΔH = -26.6 kJ/mol 17.4 Hess’s Law and Standard Enthalpies of Formation 21. Given the following data: H2(g) + ½ O2(g) → H2O(l) ΔH = -285.8 kJ N2O5(g) + H2O(l) → 2HNO3(l) ΔH = -76.6 kJ ½ N2(g) + 3/2 O2(g) + ½ H2(g) → HNO3(l) ΔH = -174.1 kJ Calculate the ΔH for the reaction 2N2(g) + 5O2(g) → 2N2O5(g) ΔH = 28.4 kJ 22. Use the values of ΔHof from the Appendix A handout to calculate the ΔHo for this reaction: SiCl4(l) + 2H2O(l) → SiO2(s) + 4HCl(aq) ΔHo = - 23 kJ 23. Use the values of ΔHof from the Appendix A handout to calculate the ΔHo for this reaction: (balance first!) 4 NH3(g) + 7 O2(g) → 4 NO2(g) + 6 H2O(l) ΔHo = - 1396 kJ