Thermochemistry Practice Problems: Specific Heat & Enthalpy
advertisement
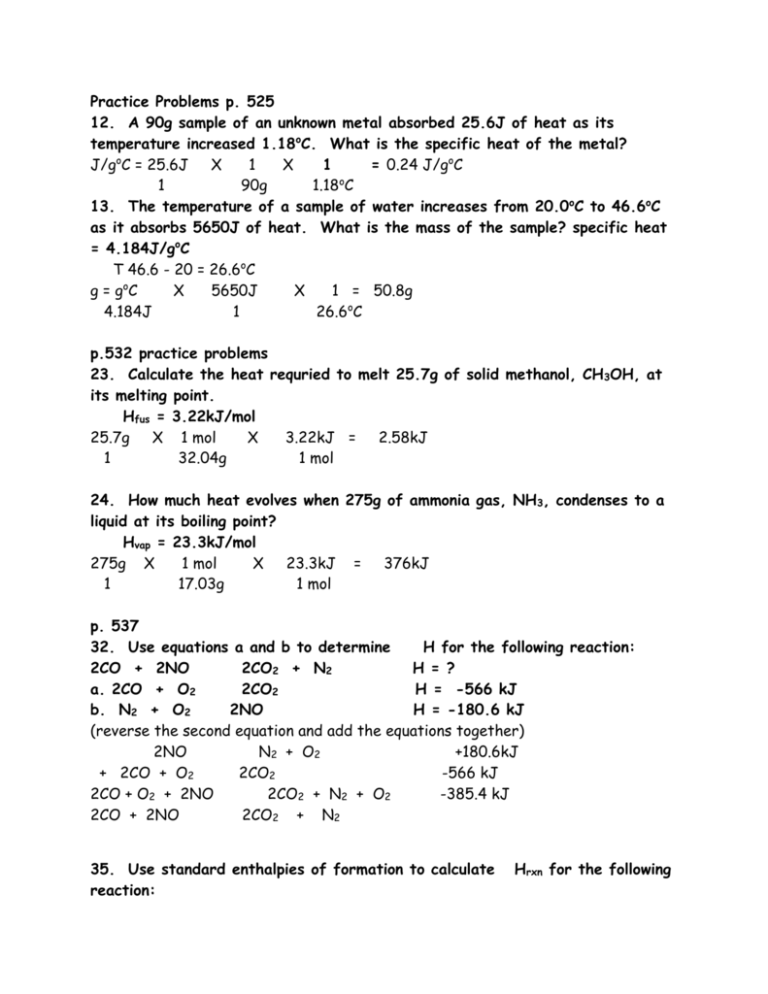
Practice Problems p. 525 12. A 90g sample of an unknown metal absorbed 25.6J of heat as its temperature increased 1.18oC. What is the specific heat of the metal? J/goC = 25.6J X 1 X 1 = 0.24 J/goC 1 90g 1.18oC 13. The temperature of a sample of water increases from 20.0oC to 46.6oC as it absorbs 5650J of heat. What is the mass of the sample? specific heat = 4.184J/goC T 46.6 - 20 = 26.6oC g = goC X 5650J X 1 = 50.8g 4.184J 1 26.6oC p.532 practice problems 23. Calculate the heat requried to melt 25.7g of solid methanol, CH3OH, at its melting point. Hfus = 3.22kJ/mol 25.7g X 1 mol X 3.22kJ = 2.58kJ 1 32.04g 1 mol 24. How much heat evolves when 275g of ammonia gas, NH3, condenses to a liquid at its boiling point? Hvap = 23.3kJ/mol 275g X 1 mol X 23.3kJ = 376kJ 1 17.03g 1 mol p. 537 32. Use equations a and b to determine H for the following reaction: 2CO + 2NO 2CO2 + N2 H = ? a. 2CO + O2 2CO2 H = -566 kJ b. N2 + O2 2NO H = -180.6 kJ (reverse the second equation and add the equations together) 2NO N 2 + O2 +180.6kJ + 2CO + O2 2CO2 -566 kJ 2CO + O2 + 2NO 2CO2 + N2 + O2 -385.4 kJ 2CO + 2NO 2CO2 + N2 35. Use standard enthalpies of formation to calculate reaction: Hrxn for the following 4NH3(g) + 7O2(g) 4NO2(g) + 6H2O(l) Hf kJ/mol NH3(g) -45.9 NO2(g) 33.2 H2O(l) -285.8 ((4)(33.2) + (6)(-285.8)) - ((4)(-45.9) + (7)(0) = -1580.8 - -183.6 = -1397.2 kJ








