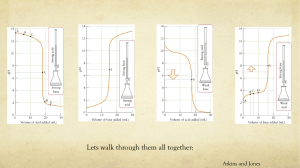
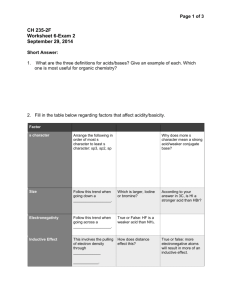
Chapter 16 Review Questions:
advertisement

Chapter 16 Review Questions: 1. Define Binary Acid. Give an example (in formula and name). 2. Define Oxyacid. Give an example (in formula and name). 3. What is the formula of acetic acid? Where is it found? 4. List 3 properties of bases. 5. Give the Traditional, Bronsted, and Lewis definitions of Acids. 6. Give the Traditional, Bronsted, and Lewis definitions of Bases. 7. Define Monoprotic and give an example of a monoprotic acid. 8. Compare Strong Acids to Weak Acids. 9. Define conjugate base. What is the conjugate base of HCl? What is the conjugate base of CH3COOH? 10. Define conjugate acid. What is the conjugate acid of OH-1? What is the conjugate acid of NH3? 11. What is neutralization? 12. How does acid strength compare to conjugate base strength? 13. Which will have a stronger conjugate base – HCl or CH3COOH. Explain. 14. Which will have a stronger conjugate acid – NH3 or NaOH? 15. Name H3PO4. 16. What ending does the acid of an –ite have? 17. Write the overall equation for Magnesium + Hydrochloric Acid -> Magnesium chloride + Hydrogen gas 18. Write the net ionic equation for the reaction in #17. 19. Calculate the molarity of NaOH if 20 mL of 1.5 M HCl reacts with 15 mL to the endpoint of the titration. 20. Calculate the volume of NaOH that would be necessary to neutralize 22 mL of 1.8 M H2SO4 with 2.2 M NaOH. 21. Which is the stronger acid – NH4+ or H2O? Explain.