Expt 4 Buffer Lab Answers
advertisement

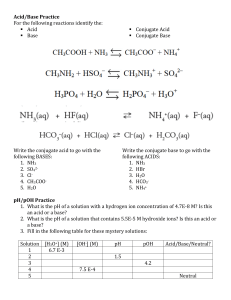
Experiment 4 Buffer Lab Answers to Application of Principles Problems (pages 4-6 to 4-8) 1. NH4NO3 is the salt that contains the conjugate acid. NH4 + is the conjugate acid of NH3. (ammonia gas in water). Write ammonia as NH3 (gas) or NH4OH A second method would be to add a small amount of strong base (NaOH) to react with NH4 + the acid and make some base. NH4 + + OH – NH3 + H2O 2. To be a buffer you must contain a weak acid and its conjugate base (as part of a salt) like HCHO2 , NaCHO2 and NaH2PO4, K2HPO4 or contain a weak base and its conjugate acid (as part of a salt) like N2H4 , N2H5Cl 3. pH = 7.73 4. pH = 4.62 5. A. pH = 8.19; 6. Kb B. new pH = 8.15 (more acidic added HCl) = 4.4 x 10 –6 to 4.5 x 10 –6 pH = 0.04