CHEM 1212 – Principles of Chemistry II
advertisement

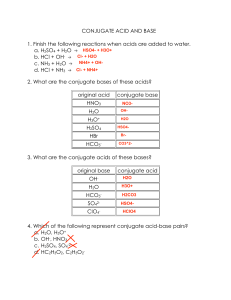
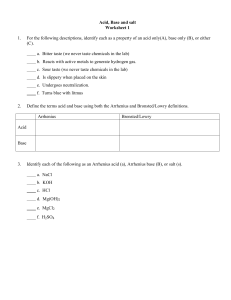
CHEM 1212 – Principles of Chemistry II, Spring 2008 Ch. 16 Homework Assignment (10 points) – Due date 3/3/08 Name:____________________________ (1) Calculate the following: (a) pH and pOH of a 0.05 M H2SO4 solution (b) [H+] and [OH-] for a 1.5 M NaOH solution (2) For each of the following aqueous reactions, identify the acid, the base, the conjugate acid and the conjugate base: (a) HNO3 + H2O (3) (b) HClO4 + CH3NH2 (c) [Cu(H2O)6]2+ + H2O NO3- + H3O+ ClO4- + CH3NH3+ [Cu(H2O)5OH]+ + H3O+ Consider the acid HA. (i) Write the acid dissociation reaction for HA. (ii) If HA is a strong acid, where will the equilibrium position be? (iii) Will Ka be small or large if HA is a strong acid? (iv) Would you expect the conjugate base of HA to be a stronger or weaker base than water? 1 (v) (4) If HA is a strong acid, what will be the major species present at equilibrium? Provide definitions of each of the following: (a) Lewis Acid / Lewis Base (b) Lowry Bronsted Acid / Lowry Bronsted Base (c) Arrhenius Acid / Arrhenius Base (5) What would happen to the pH of a sample of water if each of the following salts were dissolved in it? Provide simple explanations for each answer. (a) NaBr (b) Sr(C2H3O2)2 (c) Cu(NO3)2 (d) NH4NO2 2 (6) A 0.75 M solution of hydrofluoric acid (HF – Ka = 6.8 x10-4) is made up for a particular experiment in a General Chemistry lab, but the person making it up forgot to check the pH before he left the lab. What is the pH of this solution? 3