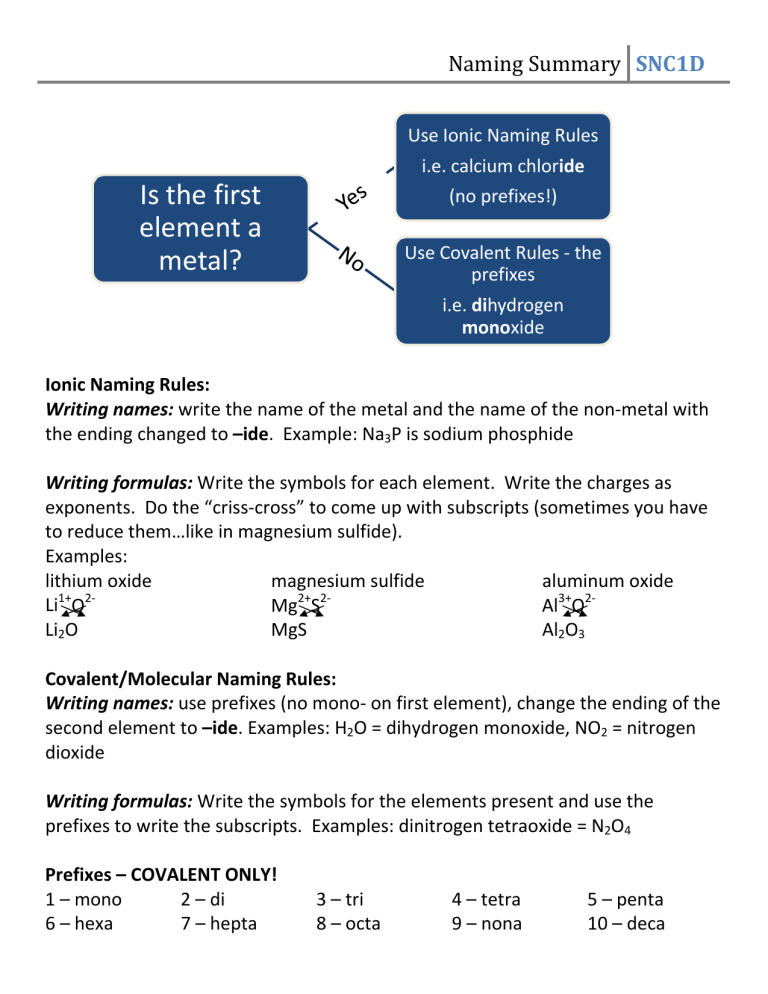
Naming Summary SNC1D Use Ionic Naming Rules i.e. calcium chloride Is the first element a metal? (no prefixes!) Use Covalent Rules ‐ the prefixes i.e. dihydrogen monoxide Ionic Naming Rules: Writing names: write the name of the metal and the name of the non‐metal with the ending changed to –ide. Example: Na3P is sodium phosphide Writing formulas: Write the symbols for each element. Write the charges as exponents. Do the “criss‐cross” to come up with subscripts (sometimes you have to reduce them…like in magnesium sulfide). Examples: lithium oxide magnesium sulfide aluminum oxide 1+ 2‐ 2+ 2‐ Li O Mg S Al3+O2‐ MgS Al2O3 Li2O Covalent/Molecular Naming Rules: Writing names: use prefixes (no mono‐ on first element), change the ending of the second element to –ide. Examples: H2O = dihydrogen monoxide, NO2 = nitrogen dioxide Writing formulas: Write the symbols for the elements present and use the prefixes to write the subscripts. Examples: dinitrogen tetraoxide = N2O4 Prefixes – COVALENT ONLY! 1 – mono 2 – di 6 – hexa 7 – hepta 3 – tri 8 – octa 4 – tetra 9 – nona 5 – penta 10 – deca Name these compounds. They may be either ionic or covalent. 1) Li2O __________________________________________ 2) PBr3 __________________________________________ 3) Na2S__________________________________________ 4) H2S __________________________________________ 5) CaO __________________________________________ 6) CF4 __________________________________________ 7) Na3N __________________________________________ 8) P2S3 __________________________________________ 9) Al2O3 __________________________________________ 10) MgO __________________________________________ Write the formulas for the following compounds. Remember, they may be either ionic or covalent compounds, so make sure you use the right method! 11) potassium oxide __________________________________________ 12) phosphorus tribromide ________________________________________ 13) calcium nitride __________________________________________ 14) dinitrogen monosulfide __________________________________________ 15) carbon monoxide __________________________________________ 16) diboron tetrahydride __________________________________________ 17) phosphorus pentabromide _____________________________________ 18) sulfur dichloride __________________________________________ 19) sodium bromide __________________________________________ 20) aluminum nitride __________________________________________ the following chemical compounds: 1) NaBr ______________________________________________ 2) CaCl2 ______________________________________________ 3) P2O5 ______________________________________________ 4) K2O ______________________________________________ 5) AlP ______________________________________________ 6) K3N ______________________________________________ 7) SO2 ______________________________________________ 8) CuOH ______________________________________________ 9) ZnO ______________________________________________ 10) V2S3 ______________________________________________ Write the formulas for the following chemical compounds: 11) silicon dioxide ______________________________________________ 12) rubidium sulfide _____________________________________________ 13) magnesium phosphide _____________________________________ 14) silver iodide ______________________________________________ 15) diboron tetrabromide __________________________________________ 16) magnesium nitride ________________________________ 17) potassium sulfide __________________________________________ 18) barium oxide ____________________________________________ 19) calcium selenide ______________________________________________ 20) carbon tetrachloride __________________________________________





