Chapter 5-3 Compound Names and Formulas
advertisement
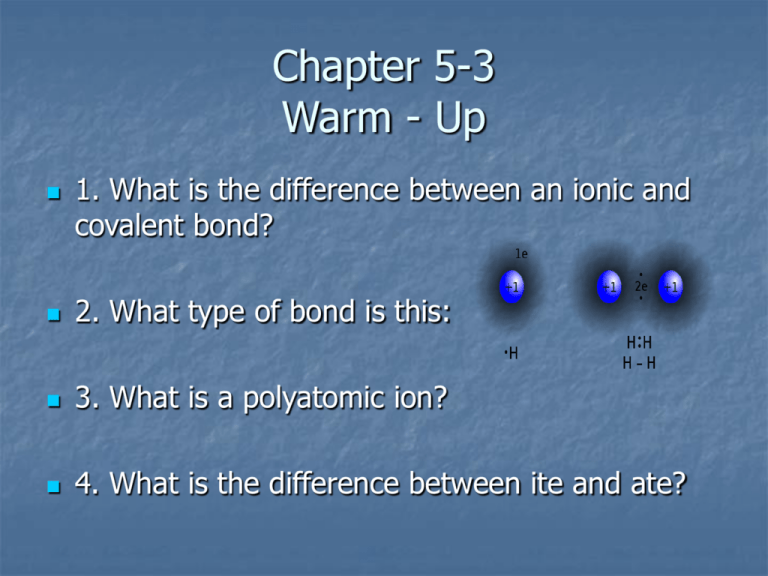
Chapter 5-3 Warm - Up 1. What is the difference between an ionic and covalent bond? 2. What type of bond is this: 3. What is a polyatomic ion? 4. What is the difference between ite and ate? Chapter 5-3 The Structure of Matter Compound Names and Formulas Common Cations The name of the cation is just like the name of the element Common Anions Anion are similar to the element in them but the ending is changed Combination of elements Determining the Charge of a Transition Metal Cation How can you tell that the iron ion in Fe2O3 has a charge of 3+? Oxide has a charge O23 x 2- = 6- charge Total positive charge must be 6+ Fe must be 3+ 2 x 3+ = 6+ Writing Formulas for Ionic Compounds What is the chemical formula for aluminum fluoride? 1. Aluminum ion: Al3+ 2. Fluoride ion: F3. Al3+F4. Least common multiple of 3 and 1 = 3 5. You need 3 positive and 3 negative charges. 6. AlF3 Practice Write the formulas for the following ionic compounds (page 161): 1. 2. 3. 4. lithium oxide beryllium chloride titanium (III) nitride cobalt (III) oxide Naming Covalent Compounds For 2 element covalent compounds, numerical prefixes tell how many atoms of each element are in the molecule. Boron trifluoride - BF3 Dinitrogen tetroxide – N2O4 Empirical Formula Empirical Formula – composition of a compound in terms of the relative numbers and kinds of atoms in the simplest ratio. Used to determine what things are made of. Emeralds are made of beryl Be3Al2Si6O18 Determining Empirical Formulas If a 142 g sample of an unknown compound contains only the elements phosphorus and oxygen and is found to contain 62 g of P and 80 g of O. What is the empirical formula? Phosphorus Oxygen 62g P x 1 mol P 80 g O x 1 mol O 30.97 g P 16.00 g O = 2.0 mol P = 5.0 mol O Empirical Formula = P2O5 Molecular Formula Molecular Formula – chemical formula that shows the number and kinds of atoms in a molecule Doesn’t show arrangement