Ionic Formula Writing Lab: Chemistry Activity
advertisement
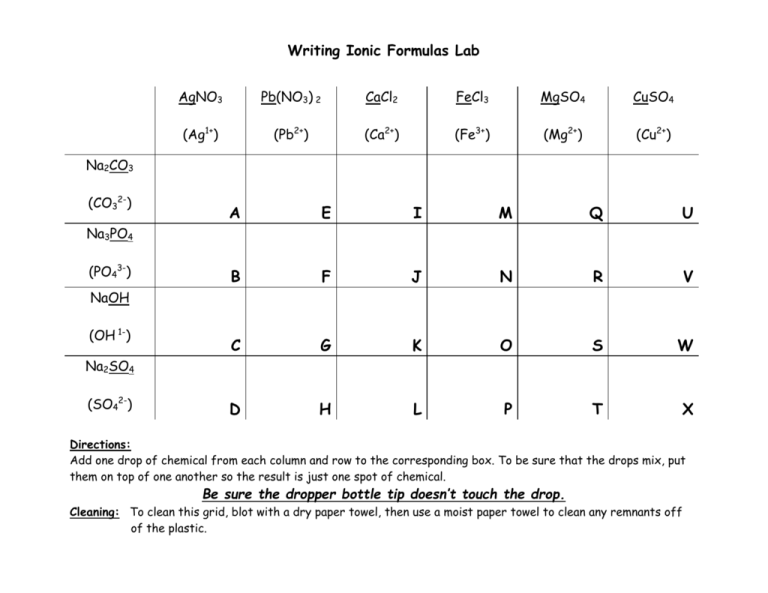
Writing Ionic Formulas Lab AgNO3 Pb(NO3) 2 CaCl2 FeCl3 MgSO4 CuSO4 (Ag1+) (Pb2+) (Ca2+) (Fe3+) (Mg2+) (Cu2+) Na2CO3 (CO32-) A E I M Q U B F J N R V C G K O S W D H L P T X Na3PO4 (PO43-) NaOH (OH 1-) Na2SO4 (SO42-) Directions: Add one drop of chemical from each column and row to the corresponding box. To be sure that the drops mix, put them on top of one another so the result is just one spot of chemical. Be sure the dropper bottle tip doesn’t touch the drop. Cleaning: To clean this grid, blot with a dry paper towel, then use a moist paper towel to clean any remnants off of the plastic. Writing Ionic Formulas Lab Activity Background When two compounds composed of ions combine, sometimes new compounds are formed. In this activity you will be combining ionic compounds and then, will predict the formulas and names of new compounds. Directions 1. Locate the chemicals and grid. 2. Drop one drop of each chemical on the corresponding square. Place drops of different chemicals directly on top of each other so the result is a larger drop on the plastic. Be sure the dropper bottle tip doesn’t touch the drop. 3. Observe the reaction and record the physical properties. (Color, opacity) 4. Record the formula of the new compound. 5. Record the name of the new compound. Data Table (Construct a data table on your paper modeled after this one!) Square Observation of precipitate New Formula Formula Name Ag2CO3 A Yellow Cloudy silver carbonate B











