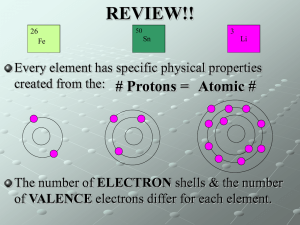
The Periodic Table
advertisement

The Periodic Table Revision guide The periodic table is a table of elements that are arranged in groups (vertical columns) and periods (horizontal rows). In each group the elements are organised so that those in the same group have similar properties. Above and below each symbol in the table there are numbers, which represent the atomic mass and the atomic number. Metals Metals make up more than 75% of the elements in the periodic table. Metals usually have most of these properties. 1. They have metallic shine. 2. They are usually solids at room temperature. 3. They are malleable. Malleable means that metals can be pressed into different shapes without breaking. 4. They are ductile meaning that they can be pulled into thin sheets or wires without breaking. 5. They are good conductors of heat and electricity. Nonmetals There are 17 nonmetals in the periodic table they have four major physical properties. 1. They rarely have metallic shine. 2. They are usually gases at room temperature. 3. Nonmetallic solids are neither malleable or ductile. 4. They are poor conductors of heat and electricity. Semi-metals The six semi-metals are B, Si, Ge, As, Sb, and Te. The properties of the semi-metals have characteristics in between metals and the nonmetals. They are good conductors of heat and electricity, but they are not good conductors or insulators. Quiz Question 1 how many nonmetals are there? 10 25 17 13 Question 2 ,which is not a characteristic of a metal? Metallic shine Malleable Insulators of heat and electricity Usually solid at room temperature Question 3 which of the following is not one of the semi-metals? B Po Ge Te Question 4 Which element has the largest mass? H Sr Mn Pt Question 5 Which of the following is not a metal? Rb Rn Be Ra