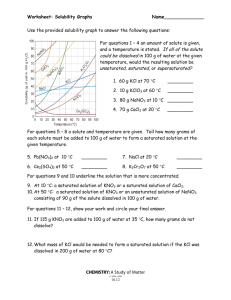
Constructing a Solubility Curve
advertisement

How much sugar can be dissolved in tea? PURPOSE: In this virtual lab, you will not be answering this question, but you will be determining how much of an ionic solute will dissolve in water. Just as a different amount of sugar will dissolve in iced tea versus hot tea, temperature can have an effect on the solubility of an ionic compound. In this lab, you will be measuring the mass of solute it takes to make a saturated solution in 100.0 g of water at various temperatures and then graphing the data using Excel. This type of graph is known as a solubility curve. You will then use your solubility curve to answer questions about the solubility of your solute. _____________________________________(your assigned solute) PROCEDURE: 1) Log on to the virtual lab at http://ir.chem.cmu.edu/irproject/applets/virtuallab/Applet_wPI.asp (You may need to load JAVA which takes a few moments). OR http://cstaff.hinsdale86.org/~kfrost Click on Chemistry Regular Click on Calendars and Help Under the Help section, click on “Solubility Lab” in the Water CD section. (You may need to load JAVA. This will take a few moments). 2) Under the FILE menu for the Irydium Chemistry Lab, click on LOAD HOMEWORK. 3) Double click on the folder SOLUBILITY AND SOLIDS. 4) Double click on the file TEMPERATURE AND THE SOLUBILITY OF SALTS You will design your own procedure as to how to collect the data you need. You MUST have at least FIVE data points across the temperature range of 0 to 100oc. You may figure out how to use the virtual lab on your own or use the following FAQs to guide you: How do I get the materials I need on the workbench to begin my experiment? Double click on the stock bottles of the materials needed. You can move them around on the workbench by dragging them with the mouse. How do I put some of the solute into the water? Once your materials are on the workbench, drag the bottle of solute to the top of the 100 mL flask of water. If you have done it properly, it should now have its cap off with a spatula in the bottle. Underneath the workbench is a space to type in the mass of solute you would like to transfer. Type in the amount, then click POUR. There is only about 130 g of solute in each jar, so if you need more than that, you will need to get another jar. How do I set the temperature? THIS IS VERY IMPORTANT!! Under the EDIT menu, click on THERMAL PROPERTIES. You MUST check the box that says INSULATED FROM SURROUNDINGS. If you notice the temperature reading on the right side of the screen (under Solution Info) fluctuating, go back and reset temperature and insulation. I don’t know why, but sometimes you must set this both before and after you add a sample of solute. How do I know if the solution is saturated? In the Solution Info at the right side of your screen, click on the button labeled SOLID (Make sure you have your clicked your cursor on the solution in the flask). If all of the solid is dissolved, it will say there is 0 grams. Is this a saturated solution? If not all of the solid dissolved, it will tell you how many grams of solid are still present. (You may want to review scientific notation). Can you figure out how much is dissolved? Is this a saturated solution? What sort of data should I collect? Look at the purpose of the experiment again to make this determination. If you have to do any calculations to the raw data to get the data you need to graph, SHOW YOUR WORK. Should I repeat the experiment? YES!! Because this is a virtual lab, your data should be reproducible. If not, you probably made an error. PROCEDURE: Write in your final procedure here. It may take a while working with the program to determine exactly what your procedure will be. DATA: Use as much or as little of this blank data table as you need. Or you may make your own. GRAPH: (attach to report) Plot temperature on the Y-axis and Mass solute on the X-axis. Use the Instruction Sheet for Excel graphing to make your graph. This is also available on the chemistry website. CALCULATIONS: (You may or may not have calculations depending on the design of your experiment.) Show any work for calculations. QUESTIONS: (Remember to answer in complete sentences). 1. How does temperature affect the solubility of your ionic solute? 2. Using your solubility curve, give an example of an unsaturated solution (mass/100.0 g water) at 35oC. 3. Using your solubility curve, give an example of a saturated solution (mass/100.0 g water) at 35oC. 4. Using your solubility curve, give an example of a supersaturated solution (mass/100.0 g water) at 35oC. 5. If you had 150.0 g water, how much of your solute would be needed to make a saturated solution at 55oC? CONCLUSION: