Semester II Exam Review Questions
advertisement

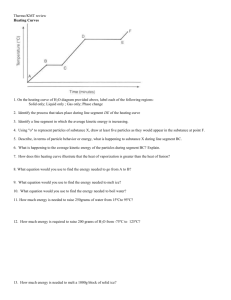
Semester II Exam Review Questions Chapter 8 Review Questions Answer the following questions. Chapters: 8, 9, 10, 11, 12.4, 13, and 15 1. How do covalent bonds form All vocabulary from this semester is fair game for the final. 2. How does the hydrogen molecule form? 3. What is a structural formula? 4. What is a lone pair? 5. How many electrons are involved in a single, double and triple covalent bond? 6. What is resonance? 7. Give examples of some compounds that break the octet rule. 8. What does VSEPR stand for and what is it? 9. What is hybridization? 10. What is a polar bond? 11. How do you decide if a bond is polar? 12. What is a polar molecule? 13. What are dispersion forces and what makes them stronger? 14. What are dipole interactions? 15. What are hydrogen bonds and what types of atoms are involved? Compound NH3 H2O PF3 Lewis Structure etally Hybridization Shape Polar or Nonpolar IMF’s CF4 Cl2CO CO32- SO2 ClO3- Name the following binary non-metal compounds. a) PBr3 a)____________________________________________ b) CO b)____________________________________________ c) N2O4 c)____________________________________________ d) CCl4 d)____________________________________________ e) SiO2 e)____________________________________________ f) BCl3 f)____________________________________________ g) CS2 g)____________________________________________ h) S2Cl2 h)____________________________________________ Write formulas for the following binary non-metal compounds. a) phosphorus pentachloride a)___________________ b) oxygen difluoride b)___________________ c) sulfur trioxide c)___________________ d) dinitrogen pentoxide d)___________________ e) silicon tetrabromide e)___________________ f) carbon dioxide f)___________________ g) boron triiodide g)___________________ h) sulfur hexafluoride h)___________________ Name the following acids. 1. H2SO3 ____________________________________________ 2. HNO3 ____________________________________________ 3. HIO4 ____________________________________________ 4. HF ____________________________________________ 5. H2SO4 ____________________________________________ 6. H3PO3 ____________________________________________ 7. HC2H3O2 ____________________________________________ 8. HClO ____________________________________________ 9. HBr ____________________________________________ Write formulas for the following acids. 1. nitrous acid ___________________ 2. phosphoric acid ___________________ 3. bromic acid ___________________ 4. hydroiodic acid ___________________ 5. hypobromous acid ___________________ 6. oxalic acid ___________________ 7. perchloric acid ___________________ 8. hydrosulfuric acid ___________________ Classifying, Finishing and Balancing reactions Balance the following equations and indicate the type of reaction taking place: 1. ____________________ 2. ____________________ ____ Ba + ____ N2 ____ Ba3N2 ____ Ca(OH)2 + ____ Al2(SO4)3 ____ CaSO4 + ____ Al(OH)3 3. ____________________ ____ K + ____ Fe2O3 ____ Fe + ____ K2O 4. ____________________ ____ C6H14 + ____ O2 ____ CO2 + ____ H2O 5. ____________________ ____ PbSO4 ____ PbO + ____ O2 + ____SO2 Classify, complete, and balance each of the following chemical equations. 6. Aqueous Calcium hydroxide combines with aqueous ammonium chloride, forming aqueous Ammonium hydroxide and aqueous Calcium Chloride. 7. Solid Sodium is combined with aqueous Zinc Iodide, forming aqueous Sodium Iodide and solid Zinc. 8. Solid Aluminum combines with gaseous chlorine, forming solid Aluminum chloride. 9. Solid Zinc and aqueous lead (II) nitrate react to form aqueous zinc nitrate and solid lead. 10. Aqueous sodium phosphate and liquid water are formed when aqueous sodium hydroxide reacts with phosphoric acid. Chemistry I: Semester Review Questions – The Mole and Stoichiometry 1. How many particles of CO2 are present in 245 g of CO2 2. How many liters of oxygen gas at STP are required to react with 65.3 g of aluminum in the production of aluminum oxide? 3. Copper reacts with silver nitrate to form silver and copper(II) nitrate. How many grams of copper are required to react with 50.0 mL of 8.0M AgNO3? 4. A substance is found to be composed of 70.58% Carbon, 5.93% Hydrogen, and 23.49% Oxygen. Find the empirical formula for the substance. Empirical Formula ____________________ 5. Given that the substance above has a molar mass of 204.24 g/mole, find the molecular formula of the substance. Show your work. Molecular formula: 6. Zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen gas. How many liters of 3.75 M HCl are needed to fully react with 165.53 g of zinc? 7. For ZnCl2, calculate the Percent Composition of each element. Data Table: The Reaction between Tin(II) Nitrate and Aluminum Mass of Empty Beaker Mass of beaker and Tin (II) Nitrate Trial 1 34.56 g 78.84 g Mass of Aluminum metal 6.45 g Mass of beaker and Tin (Sn) Metal after reaction 64.76 g 1. Balance the following Equation: _____ Sn(NO3)2 + _____ Al _____ Al(NO3)3 + _____ Sn 2. Determine the Mass of Aluminum metal and Tin(II) Nitrate used in this reaction. 3. Using the information from question (2) and the balanced equation, calculate the theoretical amount of Tin (Sn) that should have been produced from this reaction. (hint: determine the limiting reactant) Theoretical yield =_________________________, Limiting reactant = ______________ 4. Using the above data table, calculate the actual amount of Tin (Sn) produced from the reaction. 5. Using the information from questions 3 & 4, calculate the percent yield of Tin for the reaction. Based on your answer to #3 (the limiting reactant), find out how much of the excess reactant should be left at the end of the experiment. Chemistry I: Semester Review 12.4 and 15 1. List the 6 phase changes that can happen with matter. Circle the ones that are endothermic (that have to GAIN heat to make the change). Put a star by the changes that create more bonds. __________________________ _________________________ __________________ __________________________ _________________________ __________________ 2. During a phase change, the temperature does not increase, but there is a change in the amount of energy. Explain where that energy goes. Use the diagram for the element “Malack” below to answer the questions. If a question asks for a “region”, answer by giving the appropriate letter or letters. 3. In what region(s) is the temperature increasing? M I H G E 4. In what region(s) is the temperature constant? 5. In what region(s) would vaporization occur? 6. In what region(s) would freezing occur? 7. If sublimation were to occur, the element would jump from what region to what region? 8. A certain number of grams of Malack has 40 Joules of energy and they are at a temperature of 80 oC. A). What can you say for sure about the Malack? What phase(s) could it be? B). If 160 J of energy is added to the Malack, give the final temperature and phase of the Malack. 9. A certain number of grams of Malack is at 180 kJ of energy and a temperature of 280 oC. If this sample of Malack releases 110 kJ of energy, what phase change will the Malack go through? Use the diagram on the left to complete the activities and questions about the element “Yummygum”. 10. Label the regions of the diagram that correspond to the solid, liquid, and vapor phases. (Write the name of each phase directly on the graph in the appropriate regions). 11. Put a star on the point that represents the critical point for Yummygum. A). What temperature is the critical point at? B). What does it mean to be the critical point? What happens at this point? 12. Put a big circle around the triple point for Yummygum. A). What are the temperature and pressure for the triple point? B). What does it mean to be the triple point? What happens at this point? 13. What is the boiling point temperature for Yummygum when the external pressure is 75 atmospheres? 14. What is the freezing point temperature for Yummygum when the external pressure is 70 atmospheres? 15. If you were to have a container of Yummygum in your kitchen, in what state (phase of matter) would you expect it to be? Explain your answer. 16. A container of Yummygum is sitting at a pressure of 45 atm and temperature of 100 oC. What will happen as the temperature is increased 400 oC? 17. A container of Yummygum is sitting at a temperature of 100 oC and 1 atm of pressure. What phase change will occur if the pressure is increased to 90 atm? 18. Why can Yummygum not be brought to boil at a temperature of 200 oC? 19. If it is not poisonous, could Yummygum be used as a drink on earth? Explain your answer. Show work and the proper units and significance on all problems. 20. How much heat is required to warm 125 g of water from 15.0 to 47.0 oC? 21. What is the specific heat of a metal if the temperature of a 12.5-g sample increases from 19.5 oC to 33.6 oC when it absorbs 37.7 J of heat? 22. The temperature of 55.6 g of a material changes from 35.0 oC to 20.2 oC when it loses 3080 J of heat. What is its specific heat? 23. Calculate the amount of heat released, in joules, as 65.0 g of steam cools from 175 oC to -25 oC. Create a heating or cooling curve to represent the overall change. 24. A candy bar has a total mass of 55.0 grams. In a calorimetry experiment, a 2.76-g sample of this candy bar was burned in a calorimeter surrounded by 500.0 g of water. The temperature of the water in contact with the burning candy bar was measured and found to increase from an initial temperature of 22.3oC to a final temperature of 26.9oC. a. Calculate the amount of heat in calories released when the 2.76-g sample burned. b. Convert the heat in calories to nutritional Calories and then calculate the energy content (fuel value) in Cal/g. c. Calculate the total caloric content of the candy bar in Calories. ____ C10H22 + ____ O2 ____ CO2 + ____ H2O + 13483kJ 1. What volume of CO2 is produced when 45610 kJ are released at STP? 2. How many kJ of energy are produced when 67.5 grams of C10H22 is completely combusted? 3. 25.0 grams of C10H22 and 355 grams of O2 are reacted. Determine the theoretical yield of energy for this reaction. 4. Using the limiting reactant, determine the number of grams of excess reactant leftover at the end of the reaction. Chemistry I: Gas laws Semester Review Questions FOR EACH OF THE FOLLOWING, NAME THE GAS LAW AND SOLVE THE PROBLEM: 1. Hydrogen is collected over water at 0.975 atm and 28C. What is the partial pressure of H2? Water has a partial pressure of .0349 atm. 2. How many moles of chloroform, CHCl3, are required to fill a 253-mL flask at 100.0C and 940 torr? 3. You want the pressure inside a bottle to be 75.0 kPa at 23C. At what temperature in Celsius should you seal the bottle when the pressure is 1.12 atm? 4. A diver’s lungs hold about 20.0 L of air underwater at a pressure of 875 mm Hg. Assuming he holds his breath and his lungs don’t burst, what will be the volume of air in his lungs at standard pressure on the water’s surface? 5. What pressure is required to compress a gas that occupies 6500 L at 25C and 1.0 atm to a volume of 40.0 L at 18C? 6. When a canning jar is sealed at 100C the pressure inside is 101.3 kPa. What is the pressure inside the jar when it cools to room temperature, about 21C? 7. CO2 gas is collected over water at 100.3 kPa and 19C. Find the pressure of the dry gas. Water has a partial pressure of 2.23 KPa. 8. What is the temperature of a 0.00893 mol sample of neon gas that has a volume of 302 mL and a pressure of 0.941 atm? 9. A gas occupies 4.78 L at 78.1 kPa and 25C. What will the volume be at 0.975 atm and 15C? 10. A shampoo bottle contains 443 mL of air at 65C. What is its volume when it cools to 22C? 11. A balloon is filled with helium to a volume of 12.5 liters at 25C and 101 kPa. How many grams of helium are in the balloon? SOLVE THE FOLLOWING GAS STOICHIOMETRY PROBLEMS: 12. What volume of chlorine is required to produce 25.4 g of copper(II) chloride at 18C and 2.13 atm? Cu + Cl2 CuCl2 13. At 778 mm Hg and 25C, how many grams of zinc are required to produce 25.2 liters of hydrogen gas? __Zn + __HCl __ZnCl2 + __H2 14. If 5.45 g of potassium chlorate decompose, how many liters of oxygen gas are given off at 1.58 atm and 32C? 2KClO3 2KCl + 3O2 15. When aluminum is burned in 15.0 L of oxygen at 97.3 kPa and 21C, how many grams of aluminum oxide are formed? 4Al + 3O2 2Al2O3 16. If 12.8 g of CaCO3 decomposes at 38C and 0.96 atm, how many dm3 of CO2 are formed in addition to CaO? CaCO3 CaO + CO2 REMEMBER: 1cm 3 = 1 mL & 1 dm3 = 1 L Laws: P1V1T2 = P2V2T1 Ptotal = P1 + P2 + … PV = nRT R = 0.0821 atm L KPa L or R = 8.314 mol K mol K