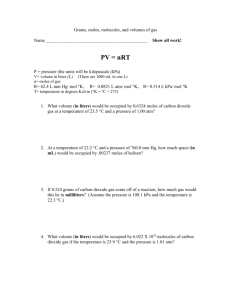
Thermochemistry Unit Review
advertisement
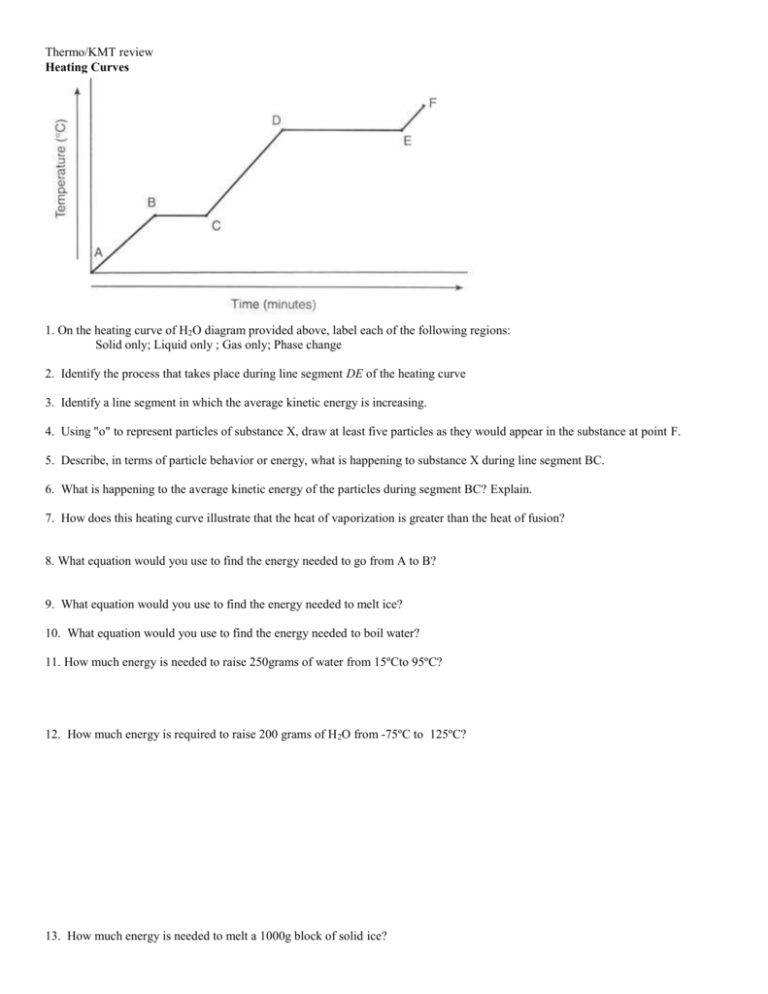
Thermo/KMT review Heating Curves 1. On the heating curve of H2O diagram provided above, label each of the following regions: Solid only; Liquid only ; Gas only; Phase change 2. Identify the process that takes place during line segment DE of the heating curve 3. Identify a line segment in which the average kinetic energy is increasing. 4. Using "o" to represent particles of substance X, draw at least five particles as they would appear in the substance at point F. 5. Describe, in terms of particle behavior or energy, what is happening to substance X during line segment BC. 6. What is happening to the average kinetic energy of the particles during segment BC? Explain. 7. How does this heating curve illustrate that the heat of vaporization is greater than the heat of fusion? 8. What equation would you use to find the energy needed to go from A to B? 9. What equation would you use to find the energy needed to melt ice? 10. What equation would you use to find the energy needed to boil water? 11. How much energy is needed to raise 250grams of water from 15ºCto 95ºC? 12. How much energy is required to raise 200 grams of H 2O from -75ºC to 125ºC? 13. How much energy is needed to melt a 1000g block of solid ice? Phase Diagrams 14. What process occurs when CO2 at 1 atm and -120ºC is heated to -40ºC? 15. What process occurs when CO2 at 100 atm and -80ºC is heated to -10ºC? 16. What process occurs when CO2 at 1 atm and 0ºC is pressurized to 100 atm? 17. What process occurs when CO2 at 0.01atm at -80ºC is pressurized to 10 atm? 18. Could liquid CO2 exist in your closet? Explain. Calorimeter, Specific Heat, and Identification of unknown metals 19. The specific heat of ethanol is 2.46 J/g-oC. Find the heat required to raise the temperature of 193 g of ethanol from 19 oC to 35oC. 20. When a 120 g sample of aluminum (Al) absorbs 9612 J of energy, its temperature increases from 25 oC to 115oC. Find the specific heat of aluminum. Be sure to include the correct unit for specific heat. 21. The specific heat of lead (Pb) is 0.129 J/g-oC. Find the amount of heat released when 2.4 mol of lead are cooled from 37.2 oC to 22.5oC. 22. How many kJ of energy are needed to raise the temperature of 165 mol of water from 10.55oC to 47.32oC? (Hint: How many J are in 1 kJ?) Using the table below answer the following questions. 23. Heat lost = heat _______ 24. An unknown metal with a mass of 25grams is heated to 99.0 oC. When it is added to 100.0grams of water with an initial temperature of 22.0 oC, the temperature of the water changed to 23.0 oC. Name the metal. 25. If 10 grams of each of the substance in the table absorb 200J of energy, which substance will have the greatest temperature change? 26. If 5 grams of copper at 200 oC are added to 100 grams of water at 22 oC, what would the final temperature of the mixture be? Pressure Conversions 27. Convert 769mmHg to atm 28. Convert 250 kPa into atm 29. Convert 5.2 atm to mmHg 30. Convert 0.89atm into kPa Boiling 31. Define boiling point (use words vapor pressure and atmospheric pressure). 32. What happens to boiling points if we increase our altitude? 33. What happens to the boiling point of water if we decrease the atmospheric pressure? Energy Stoichiometry Use the reaction below to answer the following questions ___N2 + ___H2 ____NH3 + 231 KJ 34. How much energy is released when 46.2 L of nitrogen reacts to form ammonia? 35. How much energy is released when 10.2 grams of hydrogen reacts to form ammonia? 36. How many grams of nitrogen are needed to produce 2000KJ of energy? 37. How many liters of hydrogen are needed to produce 2000KJ of energy? Finding ΔH 38. Mass of water heated: 200.5 grams Initial Temp of Water: 22.5ºC Finial Temp of Water: 35.6 ºC Mass of Propane (C3H8): 10 grams 39. Mass of water heated: 200.5 grams Initial Temp of Water: 22.5ºC Finial Temp of Water: 35.6 ºC Volume of Propane (C3H8): 2.7Liters