Ion-Ion Forces
advertisement

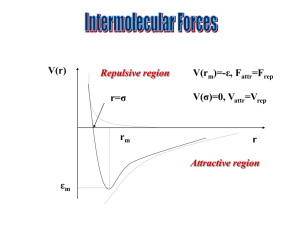
Ion-Ion Forces V = ZaZbe2/(4πЄ0Єr) Always negative as charges on ions a and b opposite, Є0Єr is the permittivity of the medium. For most gases it is close to Є0. Ion-Dipole Forces V = -Zaeµcosθ/(4πЄ0Єr2) Note – charge to account for attractive interactions. The angular dependence of the interaction – i.e. only attractive in certain directions/ r2 term. Because of cancelling of the forces due to rotation (only close attractive forces lead to net attraction because the attractive forces last longer) the forces become close range as when they are far apart cancelling is incomplete. Dipole-Dipole Forces V = - (µaµb/(4πЄ0Єr3)) x (2cosθaθb-sinθasinθb) Similar to ion-dipole. The forces are weaker because dipoles are weaker. Now there are two rotations to account for (one for each molecule). This angular dependence is very temperature dependent. As above attractive interactions at close range and will ensure longer times in these arrangements and so give attraction between molecules in all conditions. However, as temperature increases the time spent in attractive arrangements decreases due to entropy and so attractive forces decrease. This also ensures it is only when the molecules are very close that the forces are strong. The more usual form of the equation is:V = - µa2µb2/(24π2Є02Є2r6kT) K = Bolzmanns constant E.g. Calculate the potential energy at 25ºC for 2 molecules of dipole moments 3.338 x 10-30 Cm (1D) separated by 0.2nm V = - [3.338 x 10-30]4/[(24 x 3.1422)(8.854 x 10-12)2(2 x 10-10)6(1.381 x 10-23)(298)] = - 2.54 x 10-20 J (per molecule) = - 15.3 kJ mol-1 DISPERSION FORCES These exist between molecules that that have no net charge or dipole moment. Molecules which we assume are electrically neutral with a symmetric distribution of charge. This is because electrons are mobile and the electron clouds surrounding a molecule can be moved by an external field to produce a dipole. The induced dipole is given by:µind = αE where E is the field strength (Vm-1) and α is the polarizability. All molecules are polarizable. The polarizability of the molecule is related to how tightly held the electrons are by the nucleus. Increases as Z increases. Units are m3 or more usually Å3. α/ Å3 10.1 5.3 C6H6 HI 2.7 0.5 HCl HF Dipole-induced dipole forces V = - αµ/(2πЄ0Єr6) Field is determined by the dipole of the permanent dipole molecule. Dipole induced is always opposite to the permanent dipole and so always attractive. There is no angular dependence. Since electrons move faster than atoms there is no temperature dependence. Fastest molecular movement is a vibration, this takes about 10-13s and movement is about 0.01 nm. Atoms move at speed = 10-11 m/10-13 s or about 102 ms-1. Electrons in molecules move at close to relativistic speeds (i.e. they approach the speed of light, 108 ms-1) at around c/100 to c/10. Induced Dipole- Induced Dipole Forces These are also called London Dispersion Forces. V = - 3hυα/(4r6) h = Planks constant, υ = oscillation frequency of the molecule Here, instantaneous dipoles in one molecule (with no permanent dipole moment) induce an opposite dipole in another molecule resulting in an attraction. These instantaneous dipoles normally change with each molecular vibration. Summary of attractive forces All these forces are attractive. For gases we can ignore any ion forces and write that the attractive force between any molecules is given by:_ V = - (constant)/r6 Constant will depend on the molecules involved and may have contributions from all the forces. Normally permanent dipoles are stronger. The forces are always attractive. Medium range and become important over distances of 1-5 nm. Repulsive forces a) overlap of electron clouds as molecules approach b) repulsion of the positive nucleus These are very short range and are written as:V = + (constant)/r12 Total (potential) energy between molecules can be given by:V = (constant1)/r12 - (constant2)/r6 This was first described by Lennard-Jones and is known as the 6:12 potential