Stoichiometry WS

WLHS / Chem / Monson Name
Date Per
14.4 PROBLEM SET: Dalton’s Law & Graham’s Law
Part 1
: Dalton’s Law of Partial Pressures; Mole Fractions; Vapor Pressure
1) Hydrogen gas, H
2
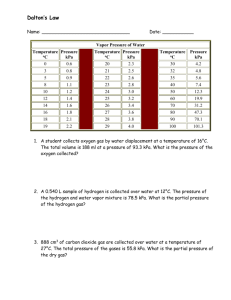
, is collected by water displacement at a total pres sure 111.0 kPa. The temperature is 50.0°C, and the partial pressure of water vapor at that temperature is 12.34 kPa.
A) What is the pressure exerted by the hydrogen gas alone?
B) The volume of hydrogen gas collected is 57.5 mL. Using your answer from (A), and the other information from the problem, calculate the amount of hydrogen gas produced (in moles and grams).
2 ) The barometer at an indoor pool reads 788 mm Hg. The temperature in the room is 30.0°C, and the partial pressure (vapor pressure) of water is 4.25 kPa. What is the partial pressure of the “dry” air (in kPa)? (HINT: subtract out the vapor pressure of water first!)
3) A mixture of gases contains 3 mol O
2
, 1 mol CO
2
, and 8 mol N
2
. Calculate the partial pressure of EACH GAS if the total pressure of the system is 0.685 atm.
4) A mixture of 28.8 g ammonia gas, NH
3
, and 6.60 g hydrogen gas, H
2
, has a total pressure of 1.35 atm. What is the partial pressure of EACH gas?
5) A cylinder contains 20.0 g O
2
at 40.0 kPa. An additional 20.0 g of neon gas is added to the container.
C) Calculate the total pressure of the gas mixture. A) Calculate the mole fraction of oxygen.
B) Calculate the mole fraction of neon. D) Calculate the partial pressure of neon.
Part 2 : Graham’s Law
6) A nitrogen molecule travels at 500.0 m/s at room temperature. What is the velocity of a helium atom at the same temperature?
7) A carbon dioxide molecule, CO
2
, travels at 45.0 m/s at a certain temperature. What is the velocity of an oxygen molecule, O
2
, at the same temperature?
8) Nitrogen gas, N
2
, effuses through an opening at a rate 2.39 times faster than an unknown gas. What is the molecular mass of the unknown gas?
9) Oxygen gas, O
2
, effuses through an opening 1.62 times faster than an unknown gas. What is the molecular mass of the unknown gas?
10) Two gases, HCl and NH
3
, are put into opposite ends of a tube simultaneously. Will the gases meet at point A,
B, or C? Explain you answer.
HCl A B C NH
3
Explanation / reasoning:
11) Hydrogen sulfide gas, H
2
S, has a very strong “rotten-egg” odor. H
2
S particles travel about 450 m/s. Methyl salicylate (C
8
H
8
O
3
) has a wintergreen odor. Benzaldehyde (C
7
H
6
O) has an almond odor. If vapors for these three substances were released at the same time from across the room, which would you smell first? Explain your answer using numerical rates calculated using Graham’s Law. If you are standing 42.0 meters away from the release point, how long will it take for you to smell each one???