Class-review
advertisement
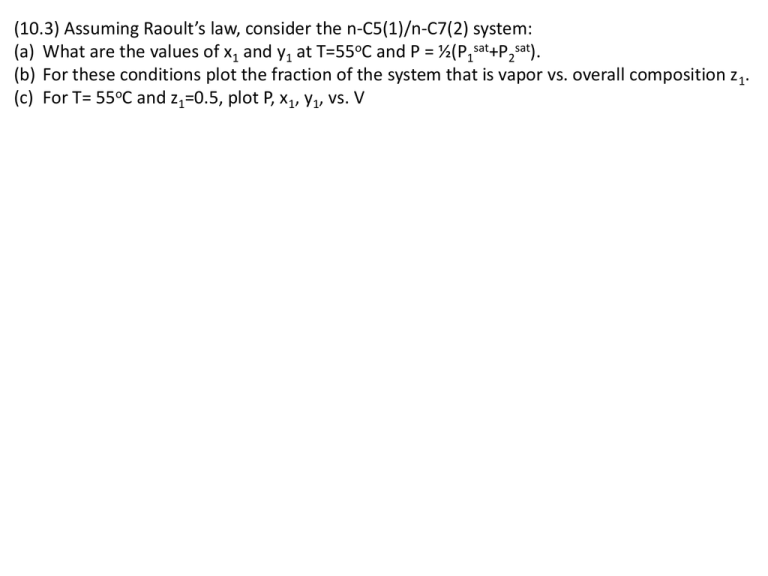
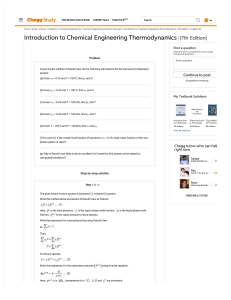
(10.3) Assuming Raoult’s law, consider the n-C5(1)/n-C7(2) system: (a) What are the values of x1 and y1 at T=55oC and P = ½(P1sat+P2sat). (b) For these conditions plot the fraction of the system that is vapor vs. overall composition z1. (c) For T= 55oC and z1=0.5, plot P, x1, y1, vs. V (10.7) A single stage L/V separation of benzene(1)/ethylbenzene(2) must produce phases of composition x1 =0.35; y1 = 0.70. Determine T and P in the separator. What additional information is needed to compute the relative amounts of L and V leaving the separator? Assume Raoult’s law applies. (10.13) A concentrated binary solution containing mostly species 2 (but x2 <1) is in equilibrium with a vapor phase containing both species 1 and 2. P =1 bar, T= 25oC. Determine good estimates of x1 and y1. H1 = 200 bar; P2sat =0.1 bar. State and justify all assumptions. (10.16) A binary consists of vapor and liquid phases in equilibrium at T. The overall mole fraction of species 1 is z1 = 0.65. At temperature T, ln g1 = 0.67 x22; ln g2 = 0.67 x12; P1sat = 32.27 kPa; P2sat =73.14 kPa. Assuming that Pyi = xigi Pisat for both components, (a) Over what range of pressures can this system exist as two phases at given T and z1? (b) For a liquid phase mole fraction x1 = 0.75, what is the P and mole fraction V? (c) Show whether or not the system exhibits an azeotrope. (11.13) The molar volume (cm3/mol) of a binary liquid mixture at T and P is given by V=120 x1 +70 x2 + (15 x1+8x2) x1 x2 (a) Find expressions for the partial molar volumes at T and P (b) Show that the expressions satisfy the additivity rule (c) Show that the expressions satisfy the Gibbs-Duhem equation (d) Show that (dV1 / dx1 ) x 1 (dV2 / dx1 ) x 0 0 (e) Plot results vs. x1. Show the pure component and the infinite dilution properties. 1 1