Name: Date: Period: MR. HOLT'S COLLEGE BOUND CHEMISTRY
advertisement

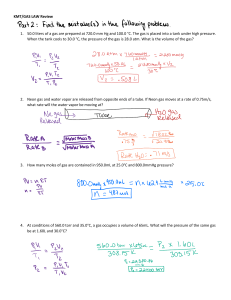
Name:_______________________________ Date: _______________________ Period: _________________ MR. HOLT’S COLLEGE BOUND CHEMISTRY HOMEWORK #3 Boyles Law: P1*V1 = P2*V2 Gay-Lussac’s Law: P1*T2 = P2*T1 Charles’ Law: V1*T2 = V2*T1 Combined Gas Law: P1*V1 = P2*V2 T1 T2 Ideal Gas Law: P*V = n*R*T R = 0.0821 L*atm mol*K 1) Match the following descriptions to the correct Gas Law _______ Can be used when Temperature is constant _______ Can be used when Pressure is constant _______ The constant for this Gas Law is always 0.0821 L*atm mol*K _______ Can be used when Pressure, Volume, and Temperature are changing _______ Can be used when Volume is constant a) Gay-Lussac’s Law b) Boyle’s Law c) Ideal Gas Law d) Charles’ Law e)Combined Gas Law 2) A sample of oxygen gas has a volume of 150.0 mL at a pressure of 0.947 atm. The pressure is then increased to 1.000 atm with a constant temperature. What Gas Law do you need to use to solve this problem? ________________________________ What is the new volume? 3) Helium gas in a balloon occupies 2.5 L at 300.0 K. The balloon is dipped into liquid nitrogen that is at a temperature of 80.0K. What Gas Law do you need to use to solve this problem? ________________________________ What is the new volume of the balloon at this lower temperature? 4) At 395.15 K the pressure of a sample of nitrogen is 1.07 atm. The temperature is then raised to 478.15 K at constant volume. What Gas Law do you need to use to solve this problem? ________________________________ What is the new pressure of the sample? 5) Calculate the pressure exerted by 43 moles of nitrogen in a 65 L cylinder at 278.15 K. What Gas Law do you need to use to solve this problem? ________________________________ 6) What will be the volume of 111 moles of nitrogen in the stratosphere where the temperature is -57 degrees Celsius and the pressure is 4.00 atm. (Hint: don’t forget to change Celsius to Kelvin) What Gas Law do you need to use to solve this problem? ________________________________ 7) A 15 L cylinder of gas at 4.8 atm pressure at 298.15K is heated to 348.15K and compressed to 17 atm. What is the new volume? What Gas Law do you need to use to solve this problem? ________________________________