Gas Laws & Molecular Mass Problems: Chemistry Worksheet
advertisement
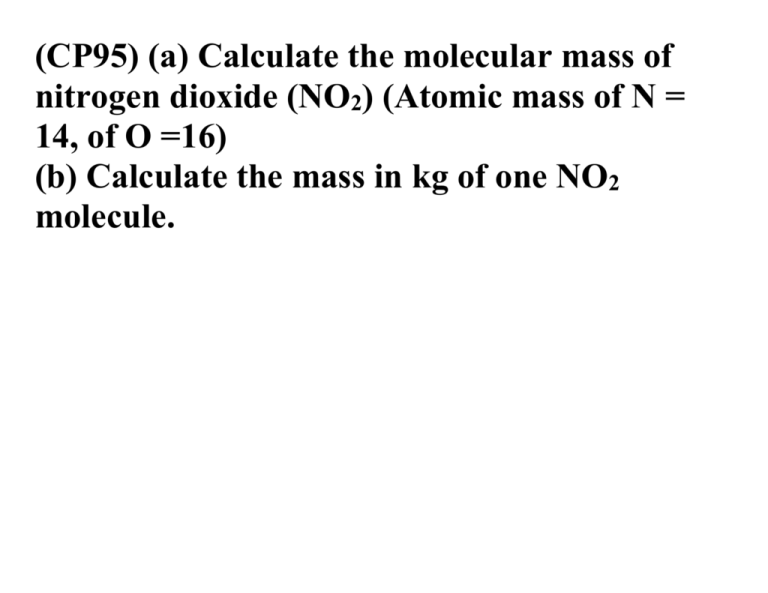
(CP95) (a) Calculate the molecular mass of nitrogen dioxide (NO2) (Atomic mass of N = 14, of O =16) (b) Calculate the mass in kg of one NO2 molecule. (CP96) The density of air at 0°C and 1.0 ATM is 1.29 kg/m3. Assuming it is entirely composed of nitrogen molecules N2, how many moles and how many molecules of N2 are there in 1.0 cc of air? (molecular mass = 28u) (CP97) Calculate the density of 3 nitrogen gas (in kg/m ) at 100°C and 1.0 ATM. (CP98) The volume of a balloon at 0°C is 2.0 L. What is its volume when the temperature rises to 100°C ? (CP99) As a result of rapid compression, the volume of a gas at 1.0 ATM pressure is reduced to half its original value and the temperature rises from 20°C to 40°C. What is the final pressure?