Lab-10-Model-Lab-1314 - Mr. Morrow's Chemistry Website
advertisement

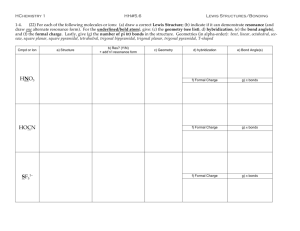
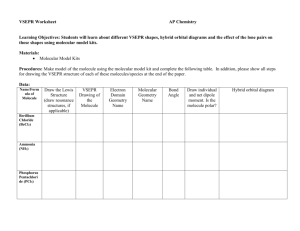
Lab 10 VSEPR Model Grade Level Indicators: Construct, interpret and apply physical and conceptual models that represent or explain systems, objects, events or concepts. Introduction Over the next four days you will be examining covalently bonded structures called molecules. On paper these molecules look very different from their true three-dimensional structures. In this lab we will practice drawing two-dimensional Lewis structures on paper and then look at these structures transformed into three dimensions using the model sets provided in the laboratory. You will then draw the molecules using three-dimensional drawing techniques (VSEPR diagrams). You will also learn about molecular geometry, hybridization and polarity. The models sets contain balls with holes that represent atoms and “sticks” that connect them that represent bonds. During this lab most atoms will follow the octet rule (Atoms in a compound lose, gain or share electrons in order to achieve a stable noble gas configuration.) Not all elements follow this rule, but most do. Hydrogen in a sense follows this rule because its electron configuration is that of the inert gas helium. Atoms that do not follow this rule in any form will always be noted in this lab in bold italic font. Examine the front cover of the model building kit. At the bottom, it sometime tells you the colors for each element. When you open the kit, there may be little white connectors which you will not be using, unless you want to show unshared electrons in a polyatomic ion. Also, there may be two shades of green “one holed balls” for the chlorine atoms. This has no chemical meaning. It is just that the kits were bought at different times and the green pigment varied from lot to lot. Also some kits have balls with more than four holes and some with less than four holes. Further reading will explain when, how and why these are used. Examine the various atom models and note the number of holes in each. When you make a model, you MUST fill up each hole with a “stick”. For example, black represents carbon and each carbon sphere has four holes. To make correct structures, you MUST fill all four holes. There is one exception to this. The pale blue atom (representing nitrogen) sometime has three or sometimes has four holes. Which do I use for nitrogen? You will have to figure that one out. By the way, carbon is always black and nitrogen is always blue. Those of you who use a blue sphere for carbon have made a mistake and, most likely, you made the mistake because you didn't read (and remember) this particular section. Sometime atoms do not follow the octet rule. Meaning some elements expand their octet and others are less than the octet. For atoms that expand their octet, you will need to use the balls (light purple and yellow) that have more than four holes. For atoms that have less than eight, you will need to use the balls (light tan) that have less than four holes. 1 Examine the light gray connectors. The short, thick ones are to be used to show single bonds. The longer, bendable ones are for double and triple bonds. You need to use two for double bonds and three for triple bonds. Do not mix long and short connectors between bond types. Some of the connectors are pear shaped and represent hybridized orbitals and can be used anytime as long as the molecule has all single bonds. Summary of the previous information: White/green/dark purple – Group 17 and Hydrogen Red or yellow – Group 2 and/or Group 16 and some transition metals as well Light tan-Group 13 Blue/Light purple- Group15 Black – Group 14 (Carbon Family) Wooden stick (wooden dowels) or shorter plastic sticks or pear shaped “sticks”-used for single bonds. Springs or longer plastic sticks are used for double and/or triple bonds. Pre Lab Questions (MUST answer in complete sentences! Chapter 8 sections 3-5 can aid you in your answer) 1. How many valence electrons does carbon have? a. How many more does it need to satisfy the octet rule? b. How many bonds are associated with carbon? c. How many holes will the balls representing carbon have? 2. How many valence electrons does oxygen and sulfur have? a. How many more does it need to satisfy the octet rule? b. How many bonds are “normally” associated with oxygen and sulfur? c. How many holes will the balls representing oxygen and sulfur have? 3. How many valence electrons does hydrogen have? a. How many more does it need to satisfy the octet rule? (Hint: H wants to look He) b. How many bonds are associated with hydrogen? c. How many holes will the balls representing hydrogen have? 4. How many valence electrons does nitrogen have? a. How many more does it need to satisfy the octet rule? b. How many bonds are normally associated with nitrogen? c. Nitrogen can have up to how many bonds? d. How many holes will the balls representing nitrogen have? 5. How many valence electrons does any halogen have? a. How many more does it need to satisfy the octet rule? b. How many bonds are normally associated with a halogen? c. How many holes will the balls representing a halogen have? 6. If you are building a model that has both hydrogen and halogens, how can you make the model to show/represent the different atoms? 7. What is used in the model kits if you want to show a single bond, double bond and a triple bond? 8. Define hybridization and how does the molecule methane explain why the molecule is symmetrical? 9. Find the definition of electron domain by using Google. Then answer the following questions. a. Does an unshared pair of electrons count as an electron domain? b. Does a single bond count in an electron domain? c. Does a double bond and triple bond count as one electron domain or multiple? 10. Some of the kits have connectors that are pear shaped, what do these pear shaped “sticks” represent? (Hint: Look at your answer to question #8) 11. What does each letter in the VSEPR theory stand for and what can it be used to predict and how? 12. How are atoms bonded on the same plane shown? “above” the paper? “behind” the paper? 2 13. List the elements in this lab that tend not to follow the rule of eight? What periodic trends can help you in predicting how and why some elements do not follow the rule of eight? 14. Find the definition of resonance in chemical bonding by using Google. Then answer the following question. a. Describe the two ways resonance can be shown on paper? b. What kind of substances tend to show this kind of bonding? 15. How can you determine the polarity of a bond between two atoms and/or the polarity of a molecule? 16. Construct data tables to record all the information required for each part. Make four separate data tables each corresponding to each procedure part during this lab. (Hint for making a data table: http://www.chem.wilkes.edu/~mencer/pdf_docs/MoleculeTable_Short.pdf) Need help with this lab try the following websites and/or your textbook. http://www.chem.ufl.edu/~myers/chm2045/shapes.htm PhET Simulation #1, PhET Simulation #2, PhET Simulation #3 Also your textbook Chapter 8.3-8.5, but page 263 will be a big help! Part I: Exploring the Shapes of Molecules Model Set. 1) Make models of each of theses structures CH4, CH2F2, NH3, HCl, H2O, NO , BF3 , SF6 , PCl5,. Draw pictures of these VSEPR diagram along with the Lewis structure. Name the hybridization around the central atom. Name the molecular geometry of each of these molecules. Note the bond angles around the central atom. Then predict which of these molecules are dipoles. (Hint: This is based on bond polarity and molecular shape!) Part II: Exploring the Inorganic Molecule Model Set. 1. Make models of each of these structures H2, Br2, O2, N2, CO2, SO2, H2O2, HCN, CH2O and draw pictures of these (VSEPR diagram) along with the Lewis structure. Name the hybridization around the central atom. Name the molecular geometry of each of these molecules. Note the bond angles around the central atom. Then predict which of these molecules are dipoles and indicate the polarity of that molecule in your report. On your VSEPR diagram insert + and + to show any dipole molecule. (Hint: Molecular polarity is based on bond polarity and molecular shape!) 2. Polyatomic ions are groups of covalently bonded group of atoms with an overall charge. Draw all (Hint: resonance structures are possible for some and also for SO2 in Part II procedure #1) Lewis structures for the ions NH4+, CO32-, CHO2-, NO3- and NO2-. Make models of each. Draw a threedimensional picture of each polyatomic ion (VSEPR diagram). Name the hybridization and the molecular geometry of each polyatomic ion. Note the bond angles around the central atom. Part III: Exploring the Organic Teacher Model Set. 1. Make a model for C2H6. Notice that the carbons spin freely around the single bond like a helicopter. Draw a VSEPR diagram. Name the hybridization, bond angle, and the molecular geometry around one of the carbon atoms. 2. Make a model for C2H4. Notice that the carbons do not spin freely around the double bond. Draw a VSEPR diagram. Name the hybridization, bond angle, and the molecular geometry around one of the carbon atoms 3 3. Now make the model for C2H2 for ethyne (more commonly called acetylene) and draw a VSEPR diagram. Name the hybridization, bond angle, and the molecular geometry around one of the carbon atoms. 4. Now make a model of ethanol, C2H5OH and draw a VSEPR diagram. Name the hybridization, bond angle, and the molecular geometry around the carbon atoms and around the oxygen atom. Circle the non-polar end and polar end of this molecule. On your VSEPR diagram insert + and + to show any dipole part of the molecule. 5. There is another possible molecule that has the same molecular formula as ethanol, C2H6O, (formula may also be written CH3OCH3) make this model and draw a VSEPR diagram. Name the hybridization, bond angle, and the molecular geometry around the carbon atoms and around the oxygen atom. 6. Now make a model of ethanoic acid, CH3COOH and draw a VSEPR diagram. Name the hybridization, bond angle, and the molecular geometry around the carbon atoms and around the oxygen atom with the attached hydrogen with a hydrogen attached. Identify the non-polar end and polar end of this molecule. BONUS Part IV: Exploring the Organic Teacher Model Set. 1. There are multiple structural and/or geometric isomers possible for the molecular formula C5H12 and for C2H2Cl2 . For one point bonus draw structural formulas for each. Put these formulas under two different headings, those for C5H12 and those for C2H2Cl2. (HINT: search structural and/or geometric isomers on the internet and you may want to try making these models, so you can see if you have made different molecules!) Part V. Post-Lab Questions 1. Explain why SO2 and CO2 have the same molecular formula, but different molecular shapes. 2. What kinds of molecular geometry, bond angles and hybridization are possible around a carbon atom? 3. How would you show bonding between calcium and iodide with a Lewis electron dot diagram? And aluminum and sulfur? (Hint: This is not like what you have been doing!!) 4. How would you show the bonding in calcium carbonate with a Lewis electron dot diagram? (Hint: This is like what you did in #4 and what you did in the lab!) 5. How is it possible to have polar bonds within a molecule, but yet the molecule is nonpolar? Use examples to explain your answer. 6. Does the presence of lone pairs of electrons (non–bonded electrons) increase or decrease the bond angle around that atom. Use an example from the models you built in this lab to support your answer. 7. Determine the molecular geometry (draw them), bond angles and hybridization of the following molecules who do not follow the octet ruleSF4 and XeF4 . (Hint visit the website: http://library.thinkquest.org/C006669/data/Chem/bonding/shapes.html ) 8. H2S and H2O have similar molecular formulas, but hydrogen sulfide is a gas at room temperature and water is a liquid. What accounts for this difference in their physical properties? 4 VSEPR Lab Grading Protocol PARTNER #1: ____________________________________ AND PARTNER #2: ____________________________________ Item Points/Out of Name & Partner /1 Date Experiment Started /1 Procedure Initialed /1 Prelab Questions /41 Part I /20 Part II /30 Part III /11 Post Lab Questions /34 Signature /1 BONUS (Structural Formulas) ( /6) BONUS (Data is neat and organized) ( /10) TOTAL /140 Special Note: I will deduct 10% off your grade for playing with the model sets; as in "Look mrmchem, at this weird structure I made" or if I see you making a structure not in the assignment. On the second occurrence, I will zero your grade. 5 1. Websites for the VSEPR lab, Lab 10, are given below Totally lost in this lab go to Website #1 , Website #2, Website #3 Help with VSEPR go to Website #1, Website #2 Help with electron domain go to Website #1 , Website #2 (Youtube video), Website #3 (e- domain and geometry) Help with resonance in chemical bonding go to Website #1, Website #2, Website #3 (definition) Help with hybridization go to Website #1, Website #2 Help with polarity of a bond and/or molecule go to Website #1, Website #2, Website #3 Help with post lab question #1 go to SO2 vs. CO2 Help with post lab question #2 go to Website #1 Help with post lab question #'s 3 and 4 got to Website #1, website for calcium ion, website for the carbonate ion, video for the carbonate ion Help with post lab question #5 go to Website #1 Help with post lab question #7 go to Website #1 Help with post lab question #8 got to: H2S vs. H2O Help with the extra credit go to Website #1 Use this website to see VSEPR models online for free at Website #1 2. Want notes or additional lectures on nomenclature go to .... Notes #1 Notes #2 Notes #3 3. Want practice quizzes on nomenclature go to .... Quiz Site #1 Quiz Site #2 Quiz Site #3 Quiz Site #4 Quiz Site #5 3. List of polyatomic ions. Some can be printed directly others are just a list that can be made into a printable list List #1 6 List #2 List #3 List #4 Data table http://www.allenisd.org/cms/lib/TX01001197/Centricity/Domain/1785/Unit%206%20Homework.pdf Structural Isomerism The general definition of isomer is: two (or more) different chemical substances that have the same molecular formula. The key point is that the substances are DIFFERENT chemicals, but have the same number of each kind of atom. There are a number of different ways to make isomers; the simplest of which is to have different connectivity. Connectivity refers to how the atoms (carbon, nitrogen, oxygen, hydrogen, etc.) in a molecule are connected to one another. For example, consider the formula C4H10. There are TWO ways to connect the four carbons. Can you figure them out before looking at the picture? The two correct isomers of C4H10 7 By the way, there are only two. Make the top structure in the picture, again if necessary, and then twist it to some other arrangement. Maybe like the one to the right? Is it a different substance than the top one in the other picture? If you say yes, what's to stop me from just twisting it right back to the original way? You cannot bend and twist a molecule to get a different connectivity. You must remove one (or more) carbons and put them SOMEWHERE ELSE (than from where they were removed) in the molecule. Here's your discussion assignment: draw all the isomers for C5H12 (there are three), C6H14 (there are five), and C7H16 (there are nine). Want more? NOOOOOOOOOOO!!!! cries the poor, befuddled chemistry student!! OK, but if you did want more, there are 18 isomers of the formula C 8H18, but you don't have to do them, just the C-4, C-5, C-6, and C-7. There is no fast and easy way to construct possible isomers. The only way is through trial-and-error. However, do not be too quick to judge that you have, say, the nine isomers of C 7H16. If one structure can be bent or twisted to exactly overlap with another, then the two models are of the same substance. There is, however, a systematic way to approach the trial-and-error. First, make the straight chain molecule, as you did above for the data table. Now, remove ONE carbon and move it to a different location. After you've exhausted the possibilities, then move TWO carbons. However, keep in mind that it gets a bit more complex. Two carbons can be moved as two one-carbon fragments or one twocarbon fragment. Keep on exhausting all the possibilities. However, one warning: if you get more isomers than I did, who do you think stands the better chance of being wrong? Do not do them in the data table format above. I suggest a title like "The nine isomers of C 7H16" and then the structures neatly done on the page. Do not draw the C-4, C-5, C-6, C-7 isomers using wedge and dashed bonds. Draw them flat, using only solid lines for bonds. You do not need to draw them in a sawtooth pattern, as the C-4 example is done in the data table. http://www.chemteam.info/Bonding/Lab-ModelBuilding/Lab-ModelBuilding-1.html 8